Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
DNA topoisomerase 2-beta
Ligand
BDBM50045000
Substrate
n/a
Meas. Tech.
ChEMBL_75890 (CHEMBL686755)
Ki
225000±n/a nM
Citation
 Mitscher, LA; Sharma, PN; Chu, DT; Shen, LL; Pernet, AG Chiral DNA gyrase inhibitors. 2. Asymmetric synthesis and biological activity of the enantiomers of 9-fluoro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-2,3-dihydro-7H- pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid (ofloxacin). J Med Chem 30:2283-6 (1987) [PubMed] Article
Mitscher, LA; Sharma, PN; Chu, DT; Shen, LL; Pernet, AG Chiral DNA gyrase inhibitors. 2. Asymmetric synthesis and biological activity of the enantiomers of 9-fluoro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-2,3-dihydro-7H- pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid (ofloxacin). J Med Chem 30:2283-6 (1987) [PubMed] Article More Info.:
Target
Name:
DNA topoisomerase 2-beta
Synonyms:
DNA topoisomerase II | DNA topoisomerase II beta | TOP2B | TOP2B_HUMAN
Type:
PROTEIN
Mol. Mass.:
183284.13
Organism:
Homo sapiens (Human)
Description:
ChEMBL_51583
Residue:
1626
Sequence:
MAKSGGCGAGAGVGGGNGALTWVTLFDQNNAAKKEESETANKNDSSKKLSVERVYQKKTQLEHILLRPDTYIGSVEPLTQFMWVYDEDVGMNCREVTFVPGLYKIFDEILVNAADNKQRDKNMTCIKVSIDPESNIISIWNNGKGIPVVEHKVEKVYVPALIFGQLLTSSNYDDDEKKVTGGRNGYGAKLCNIFSTKFTVETACKEYKHSFKQTWMNNMMKTSEAKIKHFDGEDYTCITFQPDLSKFKMEKLDKDIVALMTRRAYDLAGSCRGVKVMFNGKKLPVNGFRSYVDLYVKDKLDETGVALKVIHELANERWDVCLTLSEKGFQQISFVNSIATTKGGRHVDYVVDQVVGKLIEVVKKKNKAGVSVKPFQVKNHIWVFINCLIENPTFDSQTKENMTLQPKSFGSKCQLSEKFFKAASNCGIVESILNWVKFKAQTQLNKKCSSVKYSKIKGIPKLDDANDAGGKHSLECTLILTEGDSAKSLAVSGLGVIGRDRYGVFPLRGKILNVREASHKQIMENAEINNIIKIVGLQYKKSYDDAESLKTLRYGKIMIMTDQDQDGSHIKGLLINFIHHNWPSLLKHGFLEEFITPIVKASKNKQELSFYSIPEFDEWKKHIENQKAWKIKYYKGLGTSTAKEAKEYFADMERHRILFRYAGPEDDAAITLAFSKKKIDDRKEWLTNFMEDRRQRRLHGLPEQFLYGTATKHLTYNDFINKELILFSNSDNERSIPSLVDGFKPGQRKVLFTCFKRNDKREVKVAQLAGSVAEMSAYHHGEQALMMTIVNLAQNFVGSNNINLLQPIGQFGTRLHGGKDAASPRYIFTMLSTLARLLFPAVDDNLLKFLYDDNQRVEPEWYIPIIPMVLINGAEGIGTGWACKLPNYDAREIVNNVRRMLDGLDPHPMLPNYKNFKGTIQELGQNQYAVSGEIFVVDRNTVEITELPVRTWTQVYKEQVLEPMLNGTDKTPALISDYKEYHTDTTVKFVVKMTEEKLAQAEAAGLHKVFKLQTTLTCNSMVLFDHMGCLKKYETVQDILKEFFDLRLSYYGLRKEWLVGMLGAESTKLNNQARFILEKIQGKITIENRSKKDLIQMLVQRGYESDPVKAWKEAQEKAAEEDETQNQHDDSSSDSGTPSGPDFNYILNMSLWSLTKEKVEELIKQRDAKGREVNDLKRKSPSDLWKEDLAAFVEELDKVESQEREDVLAGMSGKAIKGKVGKPKVKKLQLEETMPSPYGRRIIPEITAMKADASKKLLKKKKGDLDTAAVKVEFDEEFSGAPVEGAGEEALTPSVPINKGPKPKREKKEPGTRVRKTPTSSGKPSAKKVKKRNPWSDDESKSESDLEETEPVVIPRDSLLRRAAAERPKYTFDFSEEEDDDADDDDDDNNDLEELKVKASPITNDGEDEFVPSDGLDKDEYTFSPGKSKATPEKSLHDKKSQDFGNLFSFPSYSQKSEDDSAKFDSNEEDSASVFSPSFGLKQTDKVPSKTVAAKKGKPSSDTVPKPKRAPKQKKVVEAVNSDSDSEFGIPKKTTTPKGKGRGAKKRKASGSENEGDYNPGRKTSKTTSKKPKKTSFDQDSDVDIFPSDFPTEPPSLPRTGRARKEVKYFAESDEEEDDVDFAMFN
Inhibitor
Name:
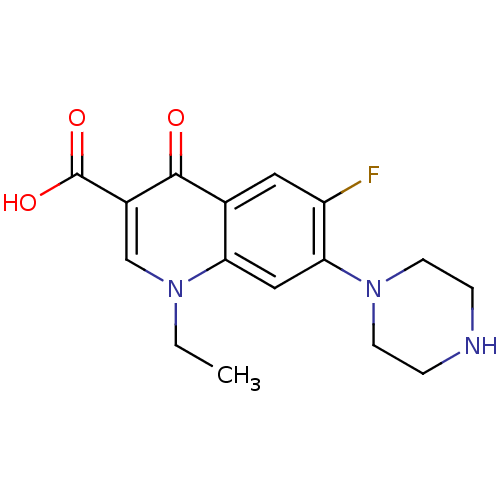
BDBM50045000
Synonyms:
(NFLX)1-Ethyl-6-fluoro-4-oxo-7-piperazin-1-yl-1,4-dihydro-quinoline-3-carboxylic acid | (norfloxacin)1-Ethyl-6-fluoro-4-oxo-7-piperazin-1-yl-1,4-dihydro-quinoline-3-carboxylic acid | 1-Ethyl-6-fluoro-4-oxo-7-piperazin-1-yl-1,4,4a,8a-tetrahydro-quinoline-3-carboxylic acid (norfloxacin) | 1-Ethyl-6-fluoro-4-oxo-7-piperazin-1-yl-1,4-dihydro-quinoline-3-carboxylic acid | 1-Ethyl-6-fluoro-4-oxo-7-piperazin-1-yl-1,4-dihydro-quinoline-3-carboxylic acid (Norfloxacin) | 1-Ethyl-6-fluoro-4-oxo-7-piperazin-1-yl-1,4-dihydro-quinoline-3-carboxylic acid(1-norfloxacin) | 1-Ethyl-6-fluoro-4-oxo-7-piperazin-1-yl-1,4-dihydro-quinoline-3-carboxylic acid(Norfloxacin) | 1-ethyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylic acid | 1-ethyl-6-fluoro-7-hexahydro-1-pyrazinyl-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid | CHEMBL9 | Chibroxin | MK-366 | NORFLOXACIN | Noroxin
Type:
Small organic molecule
Emp. Form.:
C16H18FN3O3
Mol. Mass.:
319.3308
SMILES:
CCn1cc(C(O)=O)c(=O)c2cc(F)c(cc12)N1CCNCC1