Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytoplasmic tyrosine-protein kinase BMX
Ligand
BDBM50323777
Substrate
n/a
Meas. Tech.
ChEMBL_1658703 (CHEMBL4008315)
IC50
8±n/a nM
Citation
 Liang, X; Lv, F; Wang, B; Yu, K; Wu, H; Qi, Z; Jiang, Z; Chen, C; Wang, A; Miao, W; Wang, W; Hu, Z; Liu, J; Liu, X; Zhao, Z; Wang, L; Zhang, S; Ye, Z; Wang, C; Ren, T; Wang, Y; Liu, Q; Liu, J Discovery of 2-((3-Acrylamido-4-methylphenyl)amino)-N-(2-methyl-5-(3,4,5-trimethoxybenzamido)phenyl)-4-(methylamino)pyrimidine-5-carboxamide (CHMFL-BMX-078) as a Highly Potent and Selective Type II Irreversible Bone Marrow Kinase in the X Chromosome (BMX) Kinase Inhibitor. J Med Chem 60:1793-1816 (2017) [PubMed] Article
Liang, X; Lv, F; Wang, B; Yu, K; Wu, H; Qi, Z; Jiang, Z; Chen, C; Wang, A; Miao, W; Wang, W; Hu, Z; Liu, J; Liu, X; Zhao, Z; Wang, L; Zhang, S; Ye, Z; Wang, C; Ren, T; Wang, Y; Liu, Q; Liu, J Discovery of 2-((3-Acrylamido-4-methylphenyl)amino)-N-(2-methyl-5-(3,4,5-trimethoxybenzamido)phenyl)-4-(methylamino)pyrimidine-5-carboxamide (CHMFL-BMX-078) as a Highly Potent and Selective Type II Irreversible Bone Marrow Kinase in the X Chromosome (BMX) Kinase Inhibitor. J Med Chem 60:1793-1816 (2017) [PubMed] Article More Info.:
Target
Name:
Cytoplasmic tyrosine-protein kinase BMX
Synonyms:
BMX | BMX non-receptor tyrosine kinase | BMX_HUMAN | Bone marrow tyrosine kinase gene in chromosome X protein | Cytoplasmic tyrosine-protein kinase BMX (BMX) | ETK | Epithelial and endothelial tyrosine kinase | NTK38 | Tyrosine Kinase BMX | Tyrosine-protein kinase BMX/ETK
Type:
Tyrosine-protein kinase
Mol. Mass.:
78030.42
Organism:
Homo sapiens (Human)
Description:
P51813
Residue:
675
Sequence:
MDTKSILEELLLKRSQQKKKMSPNNYKERLFVLTKTNLSYYEYDKMKRGSRKGSIEIKKIRCVEKVNLEEQTPVERQYPFQIVYKDGLLYVYASNEESRSQWLKALQKEIRGNPHLLVKYHSGFFVDGKFLCCQQSCKAAPGCTLWEAYANLHTAVNEEKHRVPTFPDRVLKIPRAVPVLKMDAPSSSTTLAQYDNESKKNYGSQPPSSSTSLAQYDSNSKKIYGSQPNFNMQYIPREDFPDWWQVRKLKSSSSSEDVASSNQKERNVNHTTSKISWEFPESSSSEEEENLDDYDWFAGNISRSQSEQLLRQKGKEGAFMVRNSSQVGMYTVSLFSKAVNDKKGTVKHYHVHTNAENKLYLAENYCFDSIPKLIHYHQHNSAGMITRLRHPVSTKANKVPDSVSLGNGIWELKREEITLLKELGSGQFGVVQLGKWKGQYDVAVKMIKEGSMSEDEFFQEAQTMMKLSHPKLVKFYGVCSKEYPIYIVTEYISNGCLLNYLRSHGKGLEPSQLLEMCYDVCEGMAFLESHQFIHRDLAARNCLVDRDLCVKVSDFGMTRYVLDDQYVSSVGTKFPVKWSAPEVFHYFKYSSKSDVWAFGILMWEVFSLGKQPYDLYDNSQVVLKVSQGHRLYRPHLASDTIYQIMYSCWHELPEKRPTFQQLLSSIEPLREKDKH
Inhibitor
Name:
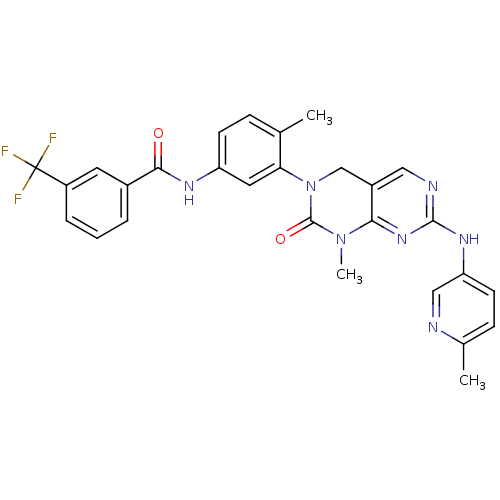
BDBM50323777
Synonyms:
CHEMBL1214141 | N-(4-Methyl-3-(1-methyl-7-(6-methylpyridin-3-ylamino)-2-oxo-1,2-dihydropyrimido[4,5-d]pyrimidin-3(4H)-yl)phenyl)-3-(trifluoromethyl)benzamide
Type:
Small organic molecule
Emp. Form.:
C28H24F3N7O2
Mol. Mass.:
547.5311
SMILES:
CN1C(=O)N(Cc2cnc(Nc3ccc(C)nc3)nc12)c1cc(NC(=O)c2cccc(c2)C(F)(F)F)ccc1C