Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Glutamate receptor 3
Ligand
BDBM50060635
Substrate
n/a
Ki
179±n/a nM
Comments
PDSP_829
Citation
 Varney, MA; Rao, SP; Jachec, C; Deal, C; Hess, SD; Daggett, LP; Lin, F; Johnson, EC; Veliçelebi, G Pharmacological characterization of the human ionotropic glutamate receptor subtype GluR3 stably expressed in mammalian cells. J Pharmacol Exp Ther 285:358-70 (1998) [PubMed]
Varney, MA; Rao, SP; Jachec, C; Deal, C; Hess, SD; Daggett, LP; Lin, F; Johnson, EC; Veliçelebi, G Pharmacological characterization of the human ionotropic glutamate receptor subtype GluR3 stably expressed in mammalian cells. J Pharmacol Exp Ther 285:358-70 (1998) [PubMed] More Info.:
Target
Name:
Glutamate receptor 3
Synonyms:
AMPA-selective glutamate receptor 3 | GLUR3 | GLURC | GRIA3 | GRIA3_HUMAN | GluR-3 | GluR-C | GluR-K3 | Glutamate receptor 3 | Glutamate receptor ionotropic AMPA | Glutamate receptor ionotropic, AMPA 3
Type:
PROTEIN
Mol. Mass.:
101172.14
Organism:
Homo sapiens (Human)
Description:
ChEMBL_468627
Residue:
894
Sequence:
MARQKKMGQSVLRAVFFLVLGLLGHSHGGFPNTISIGGLFMRNTVQEHSAFRFAVQLYNTNQNTTEKPFHLNYHVDHLDSSNSFSVTNAFCSQFSRGVYAIFGFYDQMSMNTLTSFCGALHTSFVTPSFPTDADVQFVIQMRPALKGAILSLLGHYKWEKFVYLYDTERGFSILQAIMEAAVQNNWQVTARSVGNIKDVQEFRRIIEEMDRRQEKRYLIDCEVERINTILEQVVILGKHSRGYHYMLANLGFTDILLERVMHGGANITGFQIVNNENPMVQQFIQRWVRLDEREFPEAKNAPLKYTSALTHDAILVIAEAFRYLRRQRVDVSRRGSAGDCLANPAVPWSQGIDIERALKMVQVQGMTGNIQFDTYGRRTNYTIDVYEMKVSGSRKAGYWNEYERFVPFSDQQISNDSASSENRTIVVTTILESPYVMYKKNHEQLEGNERYEGYCVDLAYEIAKHVRIKYKLSIVGDGKYGARDPETKIWNGMVGELVYGRADIAVAPLTITLVREEVIDFSKPFMSLGISIMIKKPQKSKPGVFSFLDPLAYEIWMCIVFAYIGVSVVLFLVSRFSPYEWHLEDNNEEPRDPQSPPDPPNEFGIFNSLWFSLGAFMQQGCDISPRSLSGRIVGGVWWFFTLIIISSYTANLAAFLTVERMVSPIESAEDLAKQTEIAYGTLDSGSTKEFFRRSKIAVYEKMWSYMKSAEPSVFTKTTADGVARVRKSKGKFAFLLESTMNEYIEQRKPCDTMKVGGNLDSKGYGVATPKGSALRNAVNLAVLKLNEQGLLDKLKNKWWYDKGECGSGGGDSKDKTSALSLSNVAGVFYILVGGLGLAMMVALIEFCYKSRAESKRMKLTKNTQNFKPAPATNTQNYATYREGYNVYGTESVKI
Inhibitor
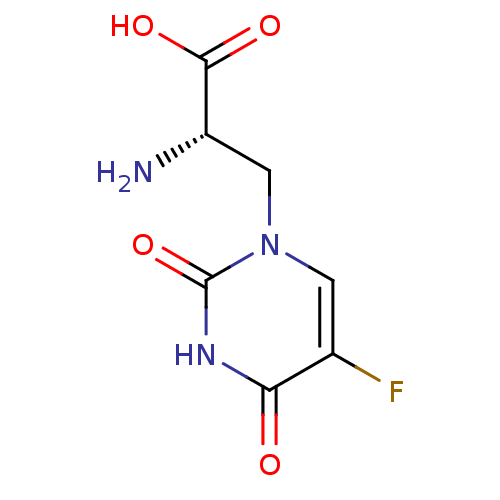
Name:
BDBM50060635
Synonyms:
(S)-2-Amino-3-(5-fluoro-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl)-propionic acid | (S)-2-amino-3-(5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)propanoic acid | (S)-5-Fluorowillardiine | 2-AMINO-3-(5-FLUORO-2,4-DIOXO-3,4-DIHYDRO-2H-PYRIMIDIN-1-YL)-PROPIONIC ACID | CHEMBL123132 | Fluorowillardiine
Type:
Small organic molecule
Emp. Form.:
C7H8FN3O4
Mol. Mass.:
217.1545
SMILES:
N[C@@H](Cn1cc(F)c(=O)[nH]c1=O)C(O)=O |r|