Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Adhesin Ata autotransporter
Ligand
BDBM281014
Substrate
n/a
Meas. Tech.
Antimicrobial Susceptibility Testing Assay
IC50
100.0±n/a nM
Citation
 Alanine, A; Beignet, J; Bleicher, K; Fasching, B; Hilpert, H; Hu, T; MacDonald, D; Jackson, S; Kolczewski, S; Kroll, C; Schaeublin, A; Shen, H; Stoll, T; Thomas, H; Wahhab, A; Zampaloni, C Peptide macrocycles against acinetobacter baumannii US Patent US10030047 Publication Date 7/24/2018
Alanine, A; Beignet, J; Bleicher, K; Fasching, B; Hilpert, H; Hu, T; MacDonald, D; Jackson, S; Kolczewski, S; Kroll, C; Schaeublin, A; Shen, H; Stoll, T; Thomas, H; Wahhab, A; Zampaloni, C Peptide macrocycles against acinetobacter baumannii US Patent US10030047 Publication Date 7/24/2018 More Info.:
Target
Name:
Adhesin Ata autotransporter
Synonyms:
ATA_ACIBT | Acinetobacter trimeric autotransporter | ata | ata:
Type:
Enzyme Catalytic Domain
Mol. Mass.:
189662.81
Organism:
Acinetobacter baumannii (strain ATCC 17978 / CIP 53.77 / LMG 1025 / NCDC KC755 / 5377)
Description:
A3M3H0
Residue:
1873
Sequence:
MNKVYKVIWNASIGAWVATSEIAKSKTKTKSKTLNLSAAVLSGVICFAPNAFAGTNTEGGIGQGTSISGTTSCREGSANTANQKDIAIGCGAQTQDRTGSNIANRNNPYNNSTGAYAGAMKQGGAISVGTGAVVEKGLGTAIGSYATTQGISGVAIGTGALSSGNTALAVGRQSAATADFSQAIGNVAAATGKGSLAIGHSATAEGYRSIAIGSPDIENADPVAGQAGAAYQPKMATKATGKDSIAFGGGAVATEENALAIGAFSESKGKKSVAIGTGAKAQKDNAVVIGDQAEASFEGGVAIGKGARSEAENSIALGKDSKASQATGESFLTKQSAPTGVLSIGDIGTERRIQNVADGAADSDAATVRQLKAARTHYVSINDNGQPGGNFENDGATGRNAIAVGVNASAAGREAMAIGGNAQAIGSGAIAMGSSSQTVGRGDVAIGRNASTQGAEGVNSNQSVAIGDQTKAIGDQSVAIGADVIAKGNSSVAIGGDDVDKIARDTELSNTYTEITGGTLQAGKYPTTEANHGSTAVGVQAVGTGAFSSAFGMTSKATGDASSAFGVMSNASGKGAAAFGAVAQATGDGASAMGINSLASGTNSTAIGSGNKPGEGANATGNSSAAIGSGAQATGDNSAAIGKGAEATNENAAAVGGGAKATGKNAAAIGGGAIADQENAVAVGQGAQSLVEGGVALGARSKVEAKNSVALGQDAVATEATGTSFLTNRDASQSNGVISVGSAGKERRITNVEDGSADSDAVTVRQLKNVDSRVNQNTSNIGKNTQNITNLNQKLDDTKTNLGNQIADTNKNLNDAKKDLGNQITDTNTKLNTTKDQLTTQINDTNTELNNTIGNTKTELNTKIDNTKTELENKGLNFAGNSGADVHRKLGDKLNIVGGAAASTPAAKTSGENVITRTTQDGIQIELLKDSKFDSVTTGNTTLNTNGLTIKEGPSITKQGINAGSKQISNVADGINAKDAVNVDQLTKVKDNLNGRITDTNNQLNDAKKDLGNQIADTNKNLNDAKKDLGNQITDTNTKLNNTKDQLTTQINDTKTELNNTIGNTKTELNSKIDSTKTELENKGLNFAGNSGADVHRKLGEKLNIIGGAAASTPAAKTSGENVITRTTKDGIQIELLKDSKFDSVTTGNTTLNTNGLTIKEGPSITKDGINASGKQITNVADGVNAKDAVNKGQLDNLAAKQNATDDAAVKYDDAKTKDKVTLKGKDGTVLDNVKAGHISSTSKEAVNGSQIHNISNSIKNSIGGNTVVNPDGSLTTNNIGGTGKNNINDAISEVKNTATKAKTTVTEGDNIVVKETVNKDGSTNYEVSTKKDLTLNSVTTGDTVLNNNGLTIKDGPSITKDGVNAGGKKITDVANGVIAQNSKDAVNGAQVHHISNSIKNSIGGNTVVNPDGSLTTNNIGGTGKNNINDAIKSVDEKVTNGVNDLTQKGLNFGANDQKTTQGKAVHRKLGDTINIVGGADAKTAEDKTSGENIITRTTEDGVKIEMLKDVKFDSVNVGGHVLNQQGLIIKGGPSITVNGINAGGKQITNVADGINAKDAVNKGQLDKQINEVKDQIGKDIGKLSDHAVQYDKDKNGNVDKSSVTLGGGEKGTNLKNVADGKVAEGSKDAVNGGQLWNVQNQVDKNSNDIKNIQNNIDNISNGKAGLVQQQKPNGEITVGRDTGGTSINMAGKEGDRVVQGVKDGEIKAGSNQAVNGGQIHKISESIKNSIGGNTTIDPKDGSITTNNIGGTGKNNINDAIGTLNQSNQELGNKITNLGDQLQQVFYDTNKRIDDVEKKANAGIAAAMALENAPFVAGKYTYAVGAAYHGGENAVGVTLRKTSDNGRWSITGGVAAASQGEPSVRVGISGVIN
Inhibitor
Name:
BDBM281014
Synonyms:
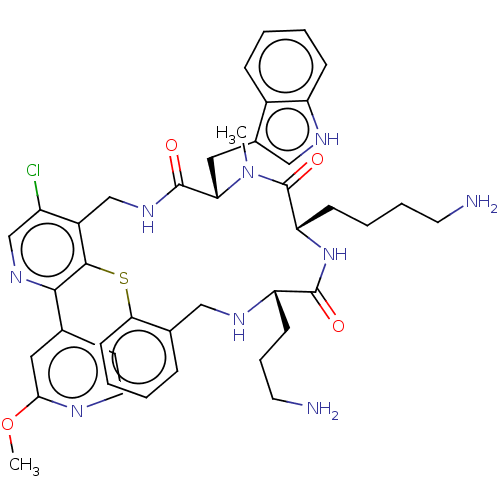
(8S,11S,14S)-8-((1H-Indol-3-yl)methyl)-11-(4-aminobutyl)-14-(3-aminopropyl)-4-chloro-1-(2-methoxypyridin-4-yl)-9-methyl-5,6,8,9,11,12,15,16-octahydrobenzo[b]pyrido[4,3-p][1,5,8,11,14]thiatetraazacycloheptadecine-7,10,13(14H)-trione | US10030047, Example 196
Type:
Small organic molecule
Emp. Form.:
C42H50ClN9O4S
Mol. Mass.:
812.422
SMILES:
COc1cc(ccn1)-c1ncc(Cl)c2CNC(=O)[C@H](Cc3c[nH]c4ccccc34)N(C)C(=O)[C@H](CCCCN)NC(=O)[C@H](CCCN)NCc3ccccc3Sc12 |r|