Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Apelin receptor
Ligand
BDBM481035
Substrate
n/a
Meas. Tech.
[35S]GTPγS Binding Assay
EC50
1.70±n/a nM
Citation
 Chen, N; Chen, Y; Debenedetto, MV; Dransfield, PJ; Harvey, JS; Heath, JA; Houze, J; Khakoo, AY; Lai, S; Ma, Z; Nishimura, N; Pattaropong, V; Swaminath, G; Yeh, W; Kreiman, C Triazole phenyl compounds as agonists of the APJ receptor US Patent US10906890 Publication Date 2/2/2021
Chen, N; Chen, Y; Debenedetto, MV; Dransfield, PJ; Harvey, JS; Heath, JA; Houze, J; Khakoo, AY; Lai, S; Ma, Z; Nishimura, N; Pattaropong, V; Swaminath, G; Yeh, W; Kreiman, C Triazole phenyl compounds as agonists of the APJ receptor US Patent US10906890 Publication Date 2/2/2021 More Info.:
Target
Name:
Apelin receptor
Synonyms:
AGTRL1 | APJ | APJ_HUMAN | APLNR | Angiotensin receptor-like 1 | Apelin receptor | Apelin receptor (APJ) | G-protein coupled receptor APJ | G-protein coupled receptor HG11
Type:
Enzyme Catalytic Domain
Mol. Mass.:
42664.06
Organism:
Homo sapiens (Human)
Description:
P35414
Residue:
380
Sequence:
MEEGGDFDNYYGADNQSECEYTDWKSSGALIPAIYMLVFLLGTTGNGLVLWTVFRSSREKRRSADIFIASLAVADLTFVVTLPLWATYTYRDYDWPFGTFFCKLSSYLIFVNMYASVFCLTGLSFDRYLAIVRPVANARLRLRVSGAVATAVLWVLAALLAMPVMVLRTTGDLENTTKVQCYMDYSMVATVSSEWAWEVGLGVSSTTVGFVVPFTIMLTCYFFIAQTIAGHFRKERIEGLRKRRRLLSIIVVLVVTFALCWMPYHLVKTLYMLGSLLHWPCDFDLFLMNIFPYCTCISYVNSCLNPFLYAFFDPRFRQACTSMLCCGQSRCAGTSHSSSGEKSASYSSGHSQGPGPNMGKGGEQMHEKSIPYSQETLVVD
Inhibitor
Name:
BDBM481035
Synonyms:
US10906890, Example 46.0
Type:
Small organic molecule
Emp. Form.:
C22H19ClF2N6O2S
Mol. Mass.:
504.94
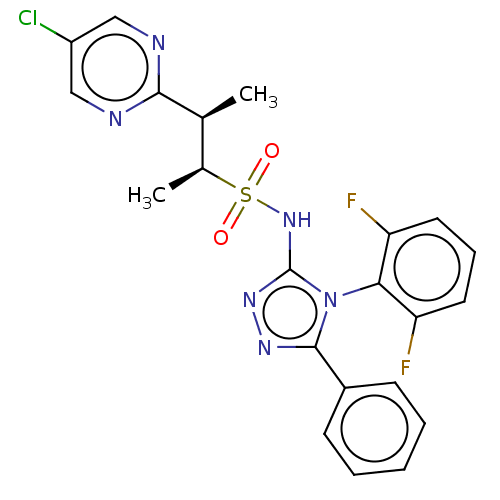
SMILES:
C[C@@H]([C@H](C)S(=O)(=O)Nc1nnc(-c2ccccc2)n1-c1c(F)cccc1F)c1ncc(Cl)cn1 |r,wU:1.0,2.2,(3.62,-3.68,;3.62,-2.14,;2.29,-1.37,;2.29,.17,;.95,-2.14,;2.29,-2.91,;-.38,-2.91,;-.38,-1.37,;-1.84,-1.85,;-2.32,-3.31,;-3.86,-3.31,;-4.34,-1.85,;-5.88,-1.85,;-6.65,-.51,;-8.19,-.51,;-8.96,-1.85,;-8.19,-3.18,;-6.65,-3.18,;-3.09,-.94,;-3.09,.6,;-4.42,1.37,;-5.76,.6,;-4.42,2.91,;-3.09,3.68,;-1.76,2.91,;-1.76,1.37,;-.42,.6,;4.96,-1.37,;6.29,-2.14,;7.62,-1.37,;7.62,.17,;8.96,.94,;6.29,.94,;4.96,.17,)|