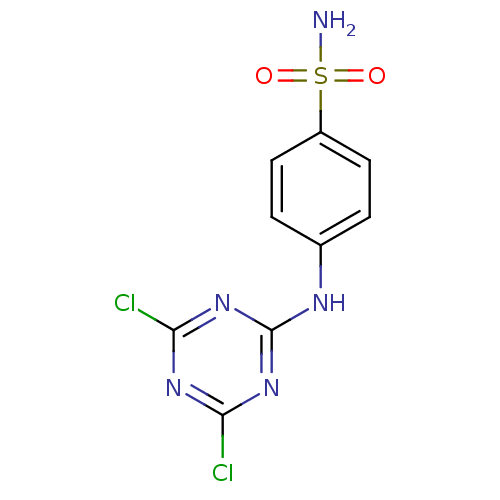
BDBM50153971 4-(4,6-Dichloro-[1,3,5]triazin-2-ylamino)-benzenesulfonamide::4-(4,6-dichloro-1,3,5-triazin-2-ylamino)benzenesulfonamide::CHEMBL366069
SMILES NS(=O)(=O)c1ccc(Nc2nc(Cl)nc(Cl)n2)cc1
InChI Key InChIKey=PUDTYTHERJACAA-UHFFFAOYSA-N
Data 10 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50153971
Found 10 hits for monomerid = 50153971
Affinity DataKi: 0.150nMAssay Description:Inhibitory activity against human carbonic anhydrase IX expressed in Escherichia coli BL21More data for this Ligand-Target Pair
Affinity DataKi: 0.150nMAssay Description:Inhibition of human recombinant CA9 by stopped flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.150nMAssay Description:Inhibition of human recombinant carbonic anhydrase 9 preincubated for 15 mins by CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 0.350nMAssay Description:Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 34.3nMAssay Description:Inhibition of human recombinant carbonic anhydrase 14 preincubated for 15 mins by CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 106nMAssay Description:Inhibition of human recombinant CA2 by stopped flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 106nMAssay Description:Inhibitory activity against human carbonic anhydrase II expressed in Escherichia coli BL21More data for this Ligand-Target Pair
Affinity DataKi: 106nMAssay Description:Inhibition of human recombinant cytosolic carbonic anhydrase 2 preincubated for 15 mins by CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 120nMAssay Description:Inhibition of human recombinant cytosolic carbonic anhydrase 1 preincubated for 15 mins by CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 120nMAssay Description:Inhibitory activity against human carbonic anhydrase I expressed in Escherichia coli BL21More data for this Ligand-Target Pair