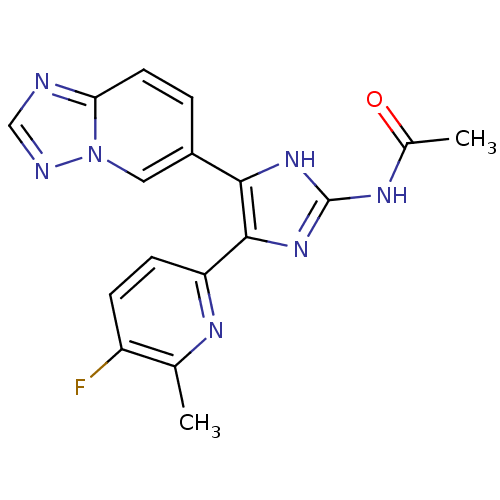
BDBM50255228 CHEMBL517068::N-(5-([1,2,4]triazolo[1,5-a]pyridin-6-yl)-4-(5-fluoro-6-methylpyridin-2-yl)-1H-imidazol-2-yl)acetamide
SMILES CC(=O)Nc1nc(c([nH]1)-c1ccc2ncnn2c1)-c1ccc(F)c(C)n1
InChI Key InChIKey=CLWJRULKTFGNLL-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50255228
Found 3 hits for monomerid = 50255228
Affinity DataKi: 2.07nMAssay Description:Binding affinity to human TGFBR1More data for this Ligand-Target Pair
Affinity DataEC50: 2.81E+4nMAssay Description:Inhibition of p38alpha (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: 111nMAssay Description:Inhibition of TGFBR1 (unknown origin) transfected in human HepG2 cells after 24 hrs by plasminogen activator inhibitor-luciferase reporter gene assayMore data for this Ligand-Target Pair