Rational inhibitor design for Pseudomonas aeruginosa salicylate adenylation enzyme PchD.
Shelton, C.L., Meneely, K.M., Ronnebaum, T.A., Chilton, A.S., Riley, A.P., Prisinzano, T.E., Lamb, A.L.(2022) J Biol Inorg Chem 27: 541-551
- PubMed: 35513576
- DOI: https://doi.org/10.1007/s00775-022-01941-8
- Primary Citation of Related Structures:
7TYB, 7TZ4 - PubMed Abstract:
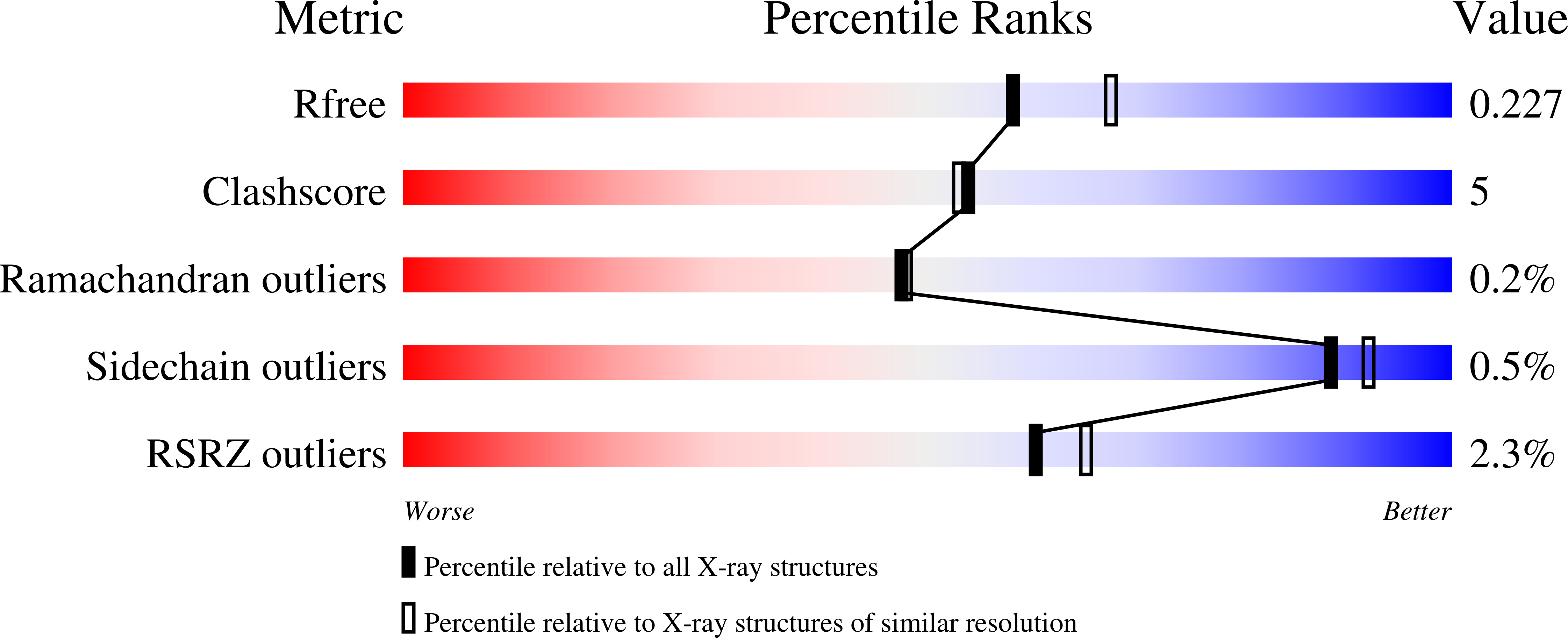
Pseudomonas aeruginosa is an increasingly antibiotic-resistant pathogen that causes severe lung infections, burn wound infections, and diabetic foot infections. P. aeruginosa produces the siderophore pyochelin through the use of a non-ribosomal peptide synthetase (NRPS) biosynthetic pathway. Targeting members of siderophore NRPS proteins is one avenue currently under investigation for the development of new antibiotics against antibiotic-resistant organisms. Here, the crystal structure of the pyochelin adenylation domain PchD is reported. The structure was solved to 2.11 Å when co-crystallized with the adenylation inhibitor 5'-O-(N-salicylsulfamoyl)adenosine (salicyl-AMS) and to 1.69 Å with a modified version of salicyl-AMS designed to target an active site cysteine (4-cyano-salicyl-AMS). In the structures, PchD adopts the adenylation conformation, similar to that reported for AB3403 from Acinetobacter baumannii.
Organizational Affiliation:
Department of Molecular Biosciences, University of Kansas, Lawrence, KS, 66045, USA.