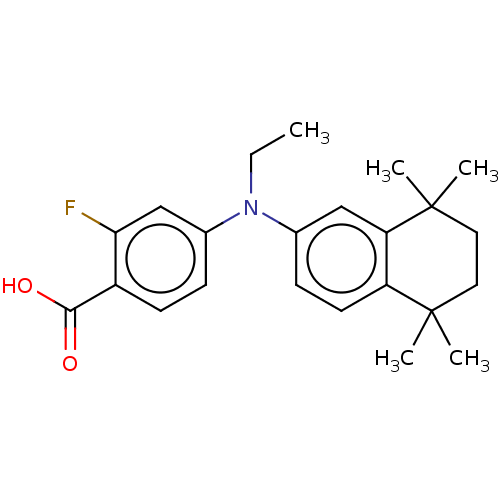
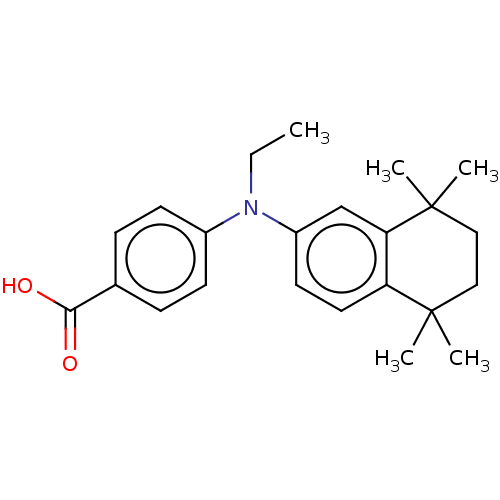
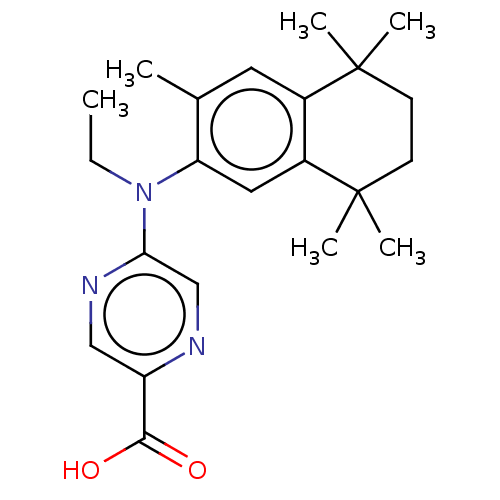
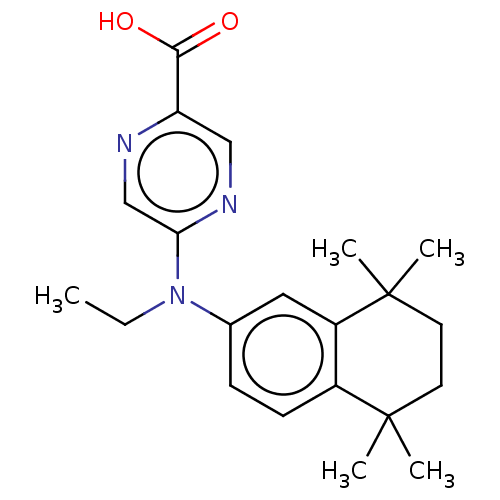
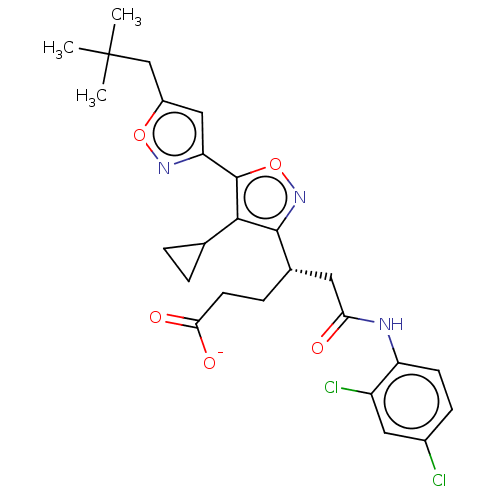
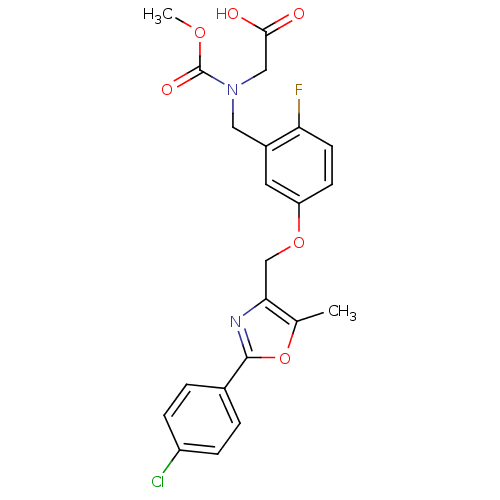
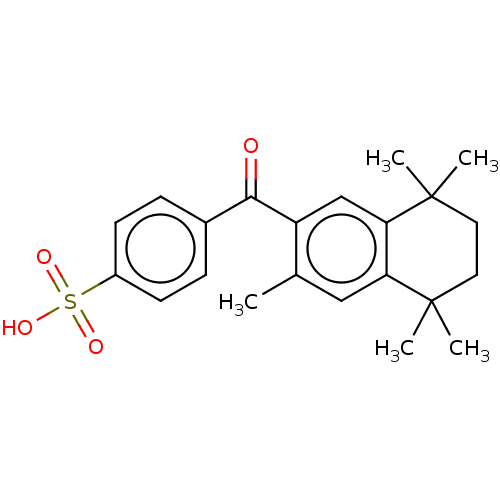
TargetRetinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataEC50: 0.130nMAssay Description:Agonist activity at RXR (unknown origin) in presence of RAR agonist Am80More data for this Ligand-Target Pair
TargetRetinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataEC50: 0.140nMAssay Description:Agonist activity at RXR (unknown origin) in presence of RAR agonist Am80More data for this Ligand-Target Pair
TargetRetinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataEC50: 7.90nMAssay Description:Agonist activity at human RXR binding domain and activation domain expressed in human HCT116 cells assessed as rexinoid activity incubated for 24 hrs...More data for this Ligand-Target Pair
TargetRetinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataEC50: 14nMAssay Description:Agonist activity at human RXR binding domain and activation domain expressed in human HCT116 cells assessed as rexinoid activity incubated for 24 hrs...More data for this Ligand-Target Pair
TargetRetinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataEC50: 14nMAssay Description:Agonist activity at human RXR binding domain and activation domain expressed in human HCT116 cells assessed as rexinoid activity incubated for 24 hrs...More data for this Ligand-Target Pair
TargetRetinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataEC50: 18nMAssay Description:Agonist activity at human RXR binding domain and activation domain expressed in human HCT116 cells assessed as rexinoid activity incubated for 24 hrs...More data for this Ligand-Target Pair
TargetRetinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataEC50: 34nMAssay Description:Agonist activity at human RXR binding domain and activation domain expressed in human HCT116 cells assessed as rexinoid activity incubated for 24 hrs...More data for this Ligand-Target Pair
TargetRetinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataEC50: 41nMAssay Description:Agonist activity at human RXR binding domain and activation domain expressed in human HCT116 cells assessed as rexinoid activity incubated for 24 hrs...More data for this Ligand-Target Pair
TargetRetinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataEC50: 52nMAssay Description:Agonist activity at human RXR binding domain and activation domain expressed in human HCT116 cells assessed as rexinoid activity incubated for 24 hrs...More data for this Ligand-Target Pair
TargetRetinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataEC50: 126nMAssay Description:Agonist activity at human RXR binding domain and activation domain expressed in human HCT116 cells assessed as rexinoid activity incubated for 24 hrs...More data for this Ligand-Target Pair
TargetRetinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataEC50: 143nMAssay Description:Agonist activity at human RXR binding domain and activation domain expressed in human HCT116 cells assessed as rexinoid activity incubated for 24 hrs...More data for this Ligand-Target Pair
TargetRetinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataEC50: 296nMAssay Description:Agonist activity at human RXR binding domain and activation domain expressed in human HCT116 cells assessed as rexinoid activity incubated for 24 hrs...More data for this Ligand-Target Pair
TargetRetinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataEC50: 364nMAssay Description:Agonist activity at human RXR binding domain and activation domain expressed in human HCT116 cells assessed as rexinoid activity incubated for 24 hrs...More data for this Ligand-Target Pair
TargetRetinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataEC50: 1.82E+4nMAssay Description:Agonist activity at human RXR binding domain and activation domain expressed in human HCT116 cells assessed as rexinoid activity incubated for 24 hrs...More data for this Ligand-Target Pair
TargetRetinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataEC50: >2.00E+4nMAssay Description:Inhibition of GAL4-fused human RXR transcriptional activity expressed in CHOK1 cells after 2 days by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetRetinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataEC50: >2.50E+4nMAssay Description:Agonist activity at human RXR by transactivation assayMore data for this Ligand-Target Pair
TargetRetinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataEC50: >8.00E+4nMAssay Description:Agonist activity at human RXR binding domain and activation domain expressed in human HCT116 cells assessed as rexinoid activity incubated for 24 hrs...More data for this Ligand-Target Pair

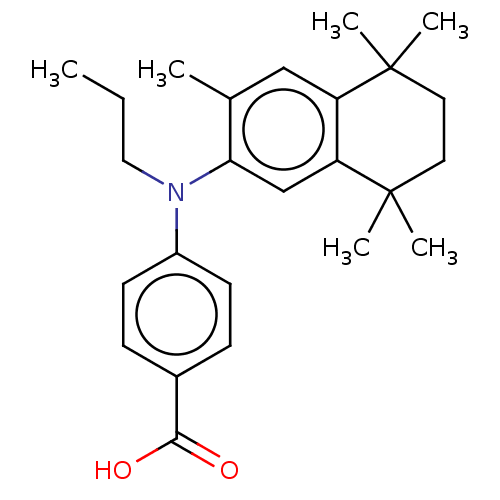
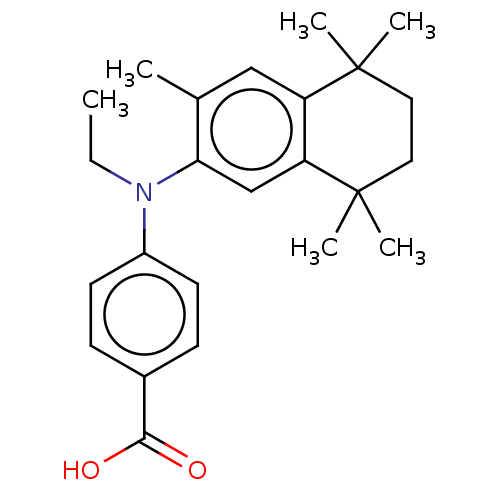
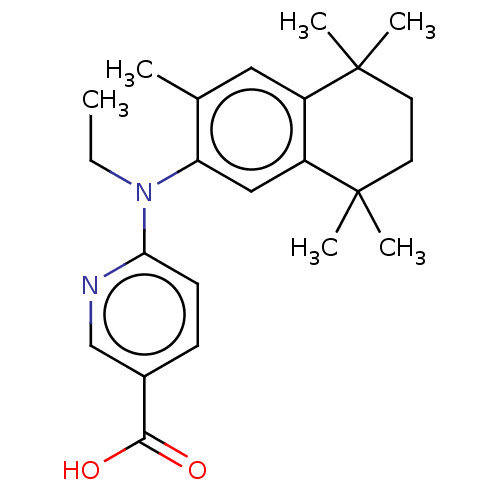
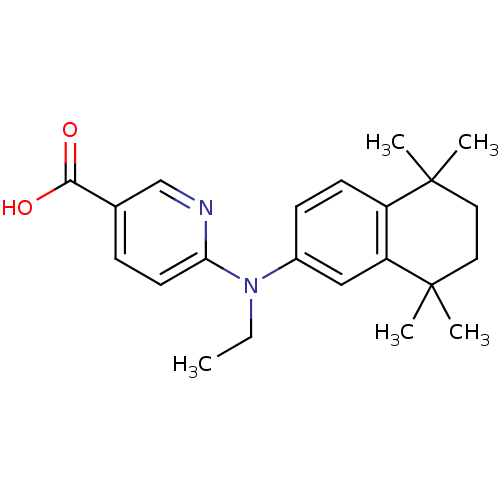
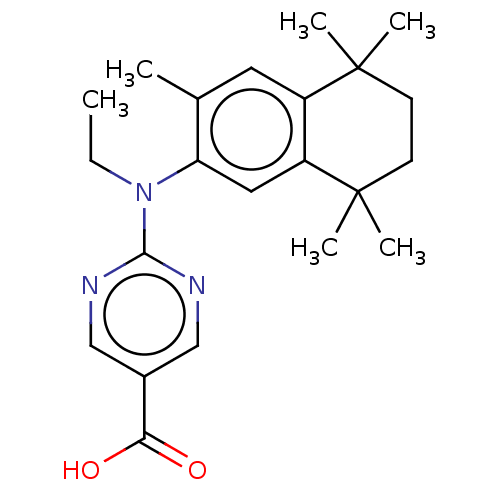
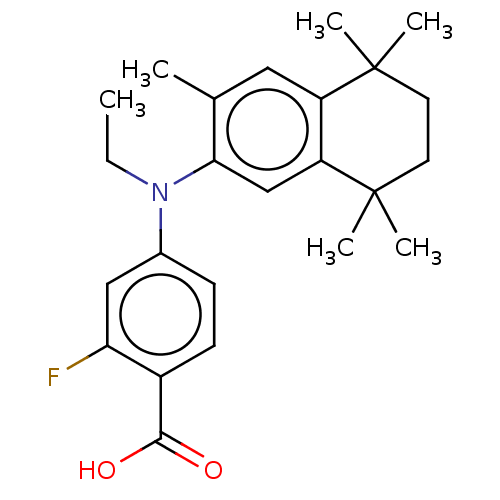
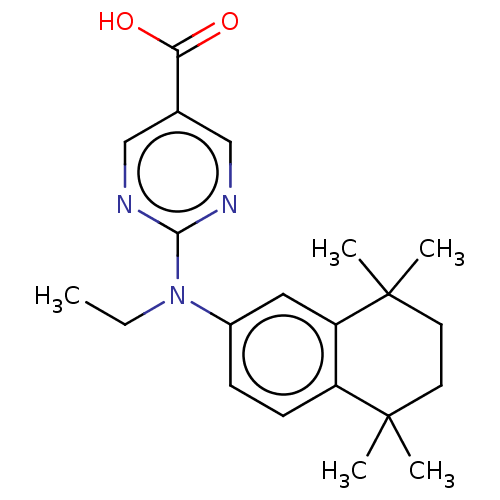
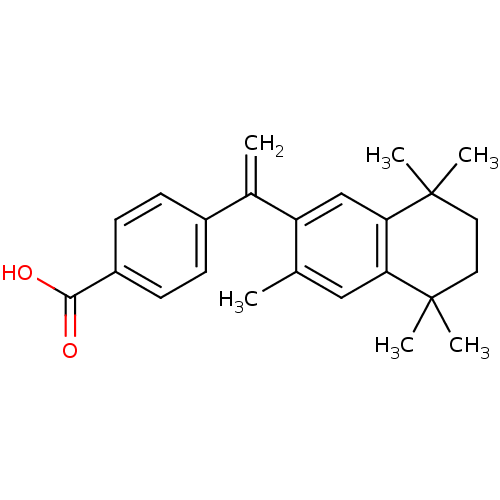
 3D Structure (crystal)
3D Structure (crystal)