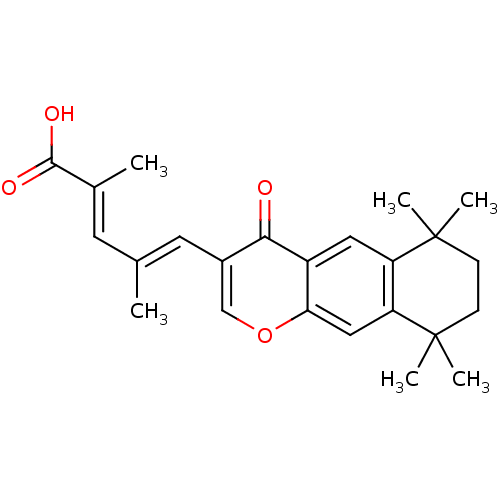
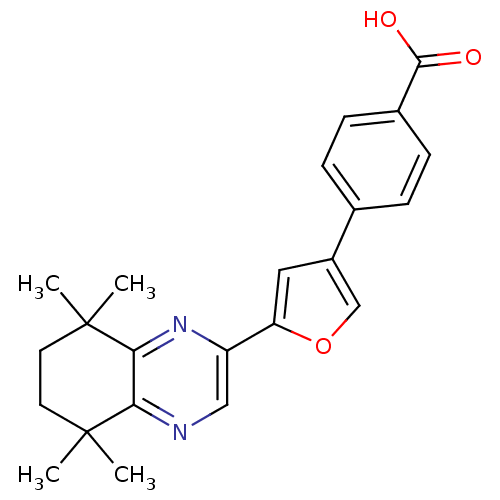
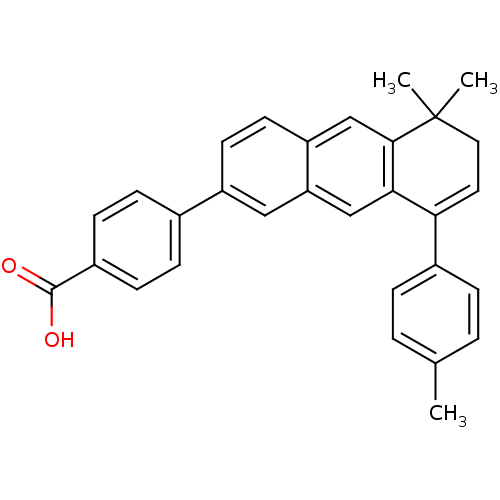
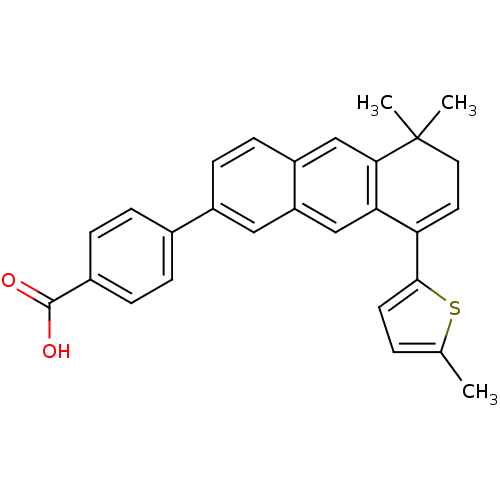
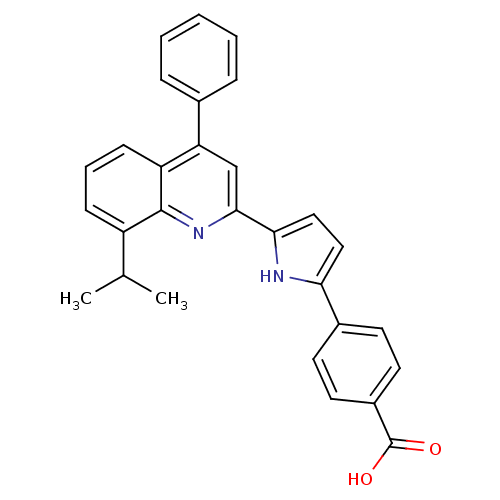
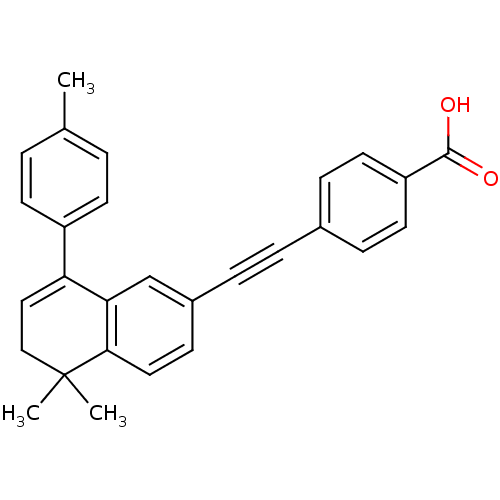
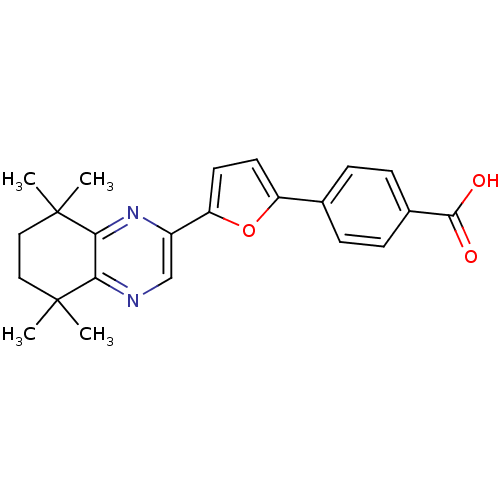
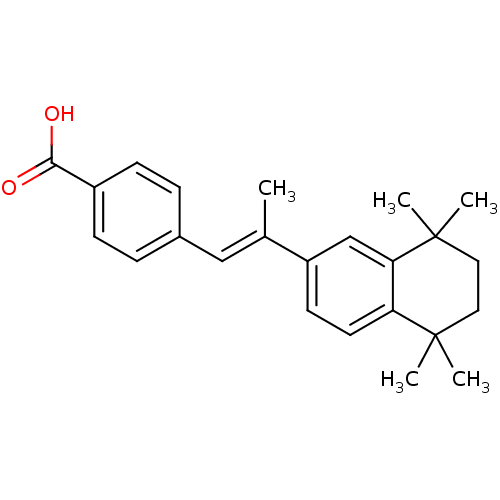
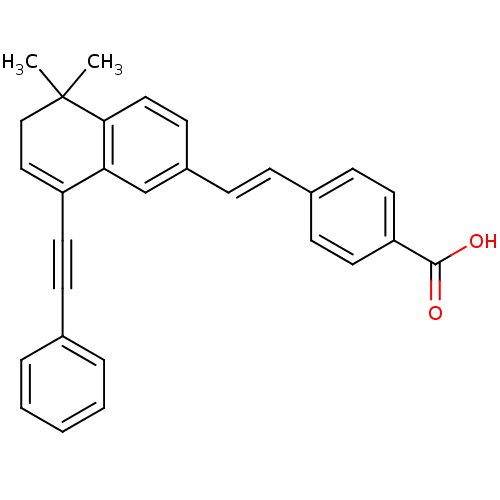
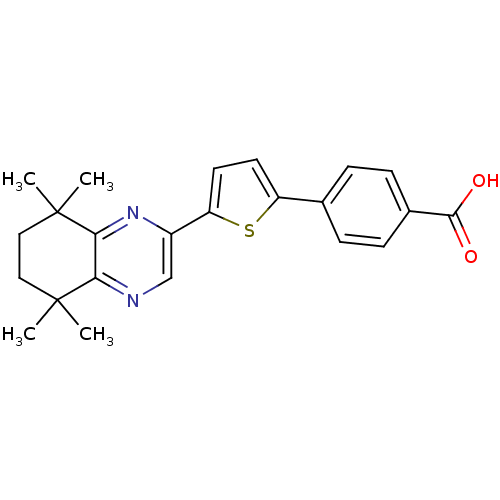
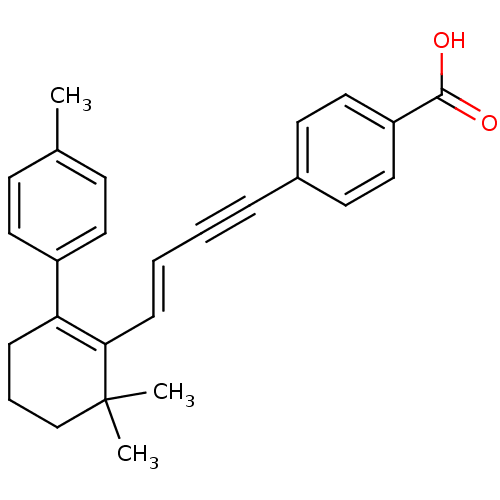
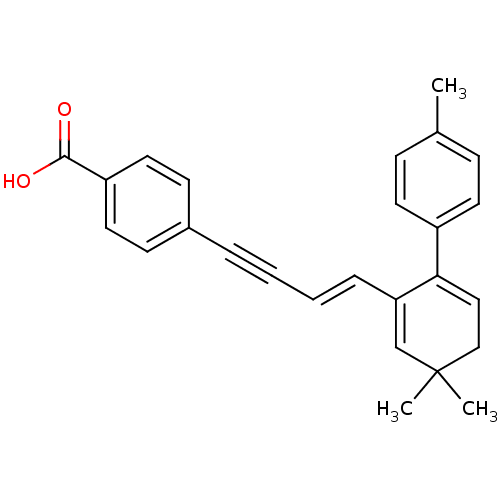
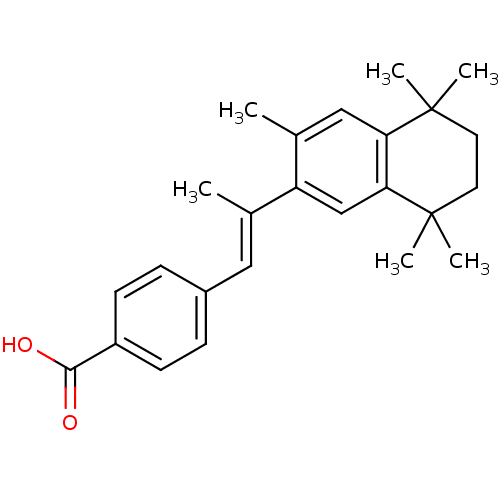
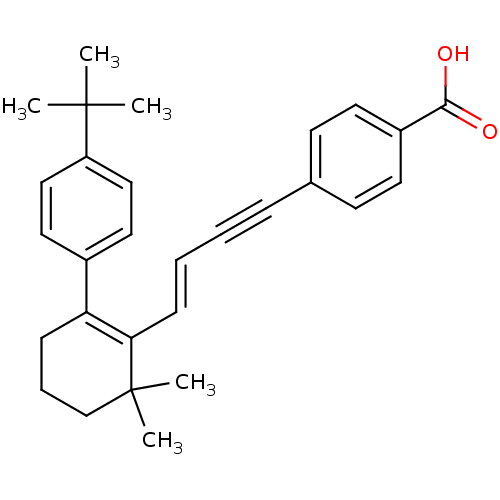
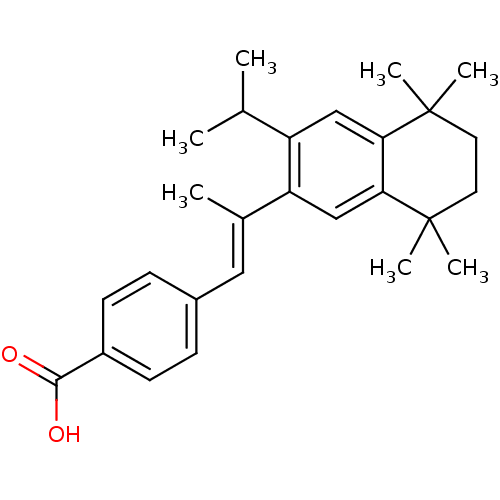
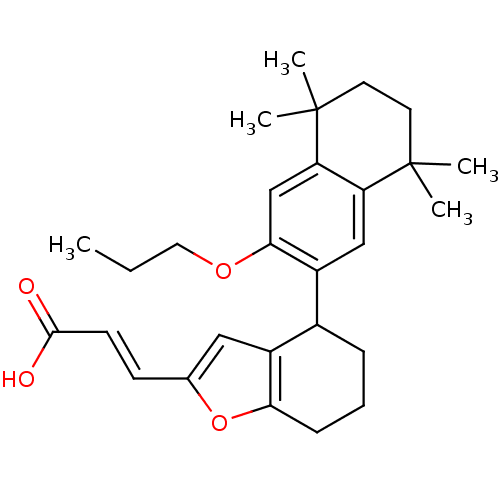
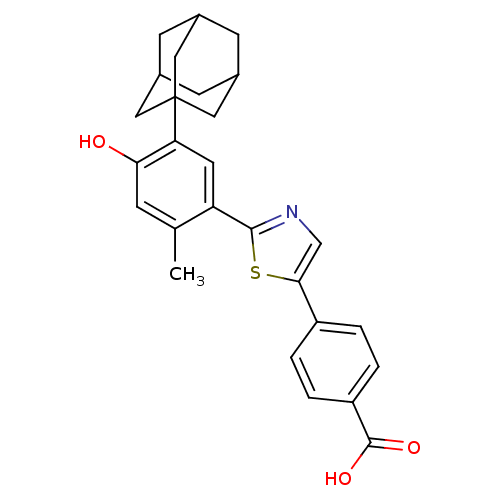
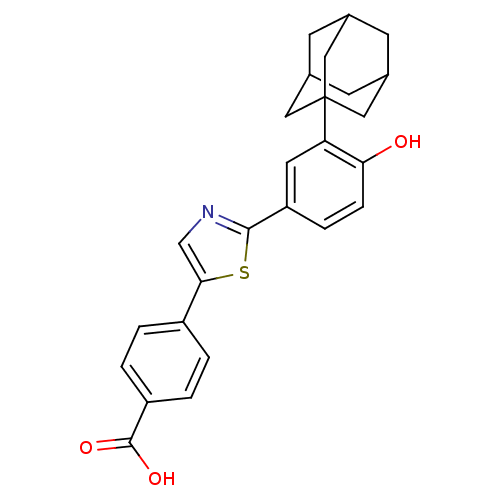
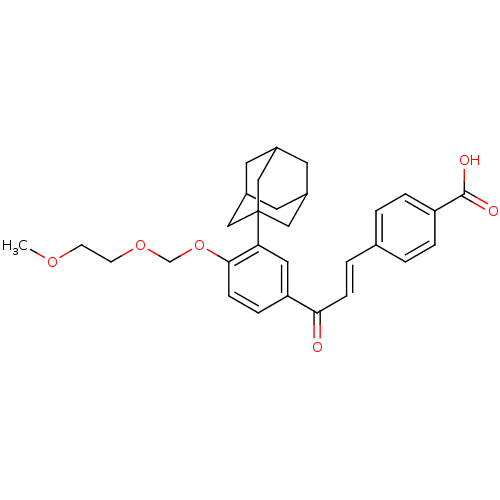
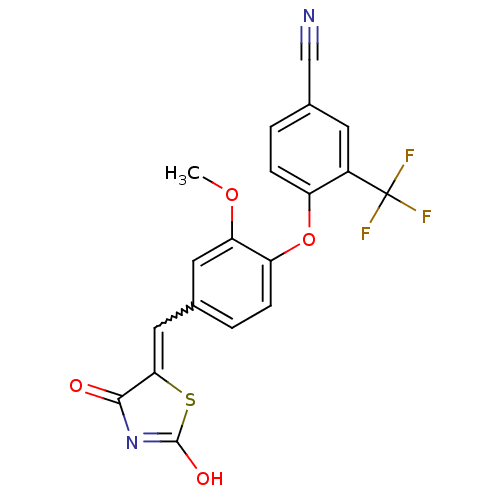
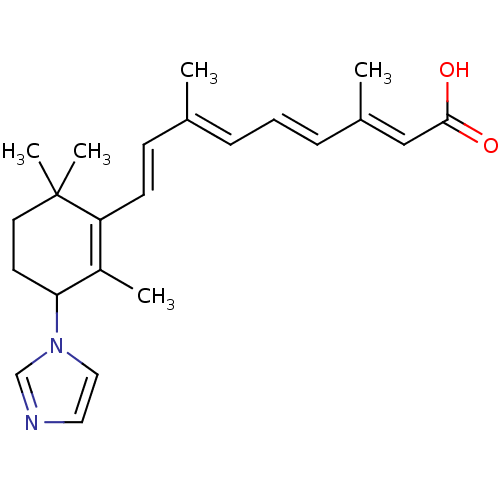
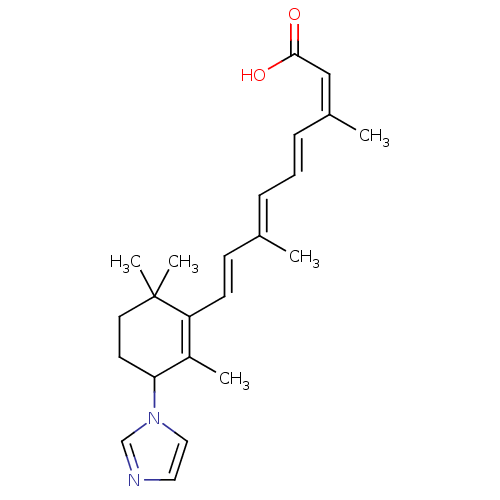
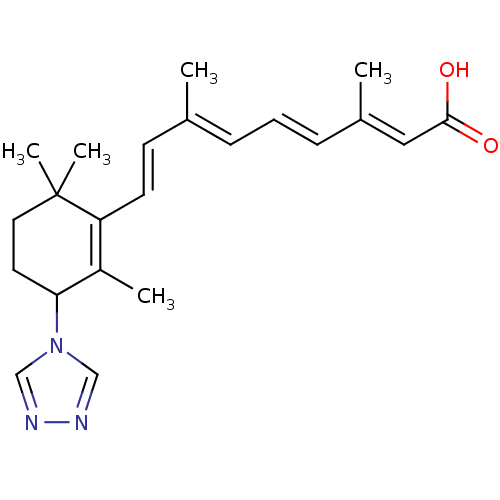
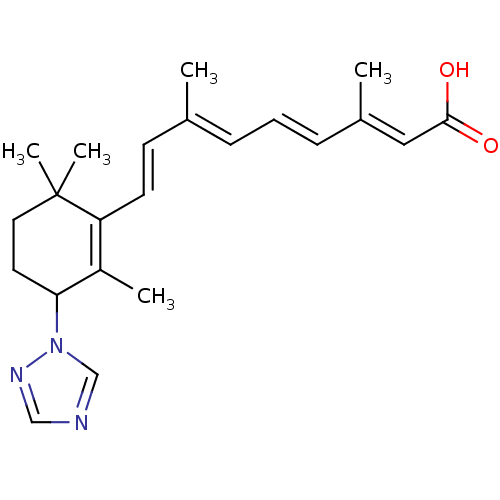
Affinity DataIC50: 0.450nMAssay Description:Binding affinity for Retinoic Acid Receptor alpha (RAR alpha)More data for this Ligand-Target Pair
Affinity DataIC50: 0.780nMAssay Description:Inhibition of [3H]-ATRA-Hl60 binding to Retinoic acid receptor alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 0.890nMAssay Description:Binding affinity for Retinoic acid receptor alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Antagonistic activity against RAR gamma in transcriptional activation assay with 3.2 nM TTNPBMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:Binding affinity for Retinoic Acid Receptor alpha (RAR alpha)More data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:Binding affinity for Retinoic Acid Receptor alpha (RAR alpha)More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Binding affinity of [3H]- RA to baculovirus expressed human RAR alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 6.5nMAssay Description:Antagonistic activity against RAR alpha in transcriptional activation assay with 32 nM TTNPBMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Binding affinity against retinoic Acid alpha receptor using [3H]- -9-cis-Retinoic Acid in competitive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Displacement of radioligand from RARalpha receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Binding affinity against retinoic Acid alpha receptor using [3H]- -9-cis-Retinoic Acid in competitive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Binding affinity for Retinoic Acid Receptor alpha (RAR alpha)More data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Antagonistic activity against RAR alpha in transcriptional activation assay with 32 nM TTNPBMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Antagonistic activity against RAR beta in transcriptional activation assay with 10 nM TTNPBMore data for this Ligand-Target Pair
Affinity DataIC50: 31nMAssay Description:Antagonistic activity of the compound was evaluated in terms of inhibition of Retinoic acid receptor alpha transactivation by ATRA (50 nM)More data for this Ligand-Target Pair
Affinity DataIC50: 31nMAssay Description:Antagonist activity of TTNPB (10 nM) function at retinoic acid receptor alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Binding affinity for Retinoic Acid Receptor alpha (RAR alpha)More data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:Binding affinity against retinoic Acid alpha receptor using [3H]- -9-cis-Retinoic Acid in competitive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Binding affinity for Retinoic Acid Receptor alpha (RAR alpha).More data for this Ligand-Target Pair
Affinity DataIC50: 41nMAssay Description:Binding affinity for Retinoic Acid Receptor alpha (RAR alpha)More data for this Ligand-Target Pair
Affinity DataIC50: 78nMAssay Description:Binding affinity for Retinoic Acid Receptor alpha (RAR alpha)More data for this Ligand-Target Pair
Affinity DataIC50: 83nMAssay Description:Binding affinity for Retinoic Acid Receptor alpha (RAR alpha)More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Binding affinity against retinoic Acid alpha receptor using [3H]- -9-cis-Retinoic Acid in competitive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 114nMAssay Description:Inverse agonist activity at RARalpha (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Binding affinity for Retinoic Acid Receptor alpha (RAR alpha)More data for this Ligand-Target Pair
Affinity DataIC50: 210nMAssay Description:Antagonist activity of TTNPB (10 nM) function at retinoic acid receptor gammaMore data for this Ligand-Target Pair
Affinity DataIC50: 220nMAssay Description:Antagonist activity of TTNPB (10 nM) function at retinoic acid receptor alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 225nMAssay Description:Antagonist activity of TTNPB (10 nM) function at retinoic acid receptor alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 638nMAssay Description:Binding affinity against retinoic Acid alpha receptor using [3H]- -9-cis-Retinoic Acid in competitive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 875nMAssay Description:Ability to inhibit TTNPB-induced transactivation at retinoic acid receptor alphaMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMAssay Description:Binding affinity against retinoic Acid alpha receptor using [3H]- -9-cis-Retinoic Acid in competitive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMAssay Description:Inhibitory concentration for lipogenesis induced by retinoic acid receptor alpha in C3H10T1/2 clone 8 fibroblast cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.28E+3nMAssay Description:Antagonist activity at Gal4-fused RARalpha (unknown origin) transfected in HEK293 cells assessed as inhibition of ATRA-induced transcriptional activi...More data for this Ligand-Target Pair
Affinity DataIC50: 2.65E+3nMAssay Description:Antagonist activity at Gal4-fused RARalpha (unknown origin) transfected in HEK293 cells assessed as inhibition of ATRA-induced transcriptional activi...More data for this Ligand-Target Pair
Affinity DataIC50: 4.47E+3nMAssay Description:Antagonist activity at Gal4-fused RARalpha (unknown origin) transfected in HEK293 cells assessed as inhibition of ATRA-induced transcriptional activi...More data for this Ligand-Target Pair
Affinity DataIC50: >8.30E+3nMAssay Description:Antagonist activity at RARalpha by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 9.00E+3nMAssay Description:Displacement of [11,12-3H]ARTA from RARalphaMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Displacement of [11,12-3H]ARTA from RARalphaMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Displacement of [11,12-3H]ARTA from RARalphaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Displacement of [11,12-3H]ARTA from RARalphaMore data for this Ligand-Target Pair
Affinity DataIC50: 4.10E+5nMAssay Description:Displacement of [11,12-3H]ARTA from RARalphaMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+6nMAssay Description:Displacement of [11,12-3H]ARTA from RARalphaMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+6nMAssay Description:Displacement of [11,12-3H]ARTA from RARalphaMore data for this Ligand-Target Pair

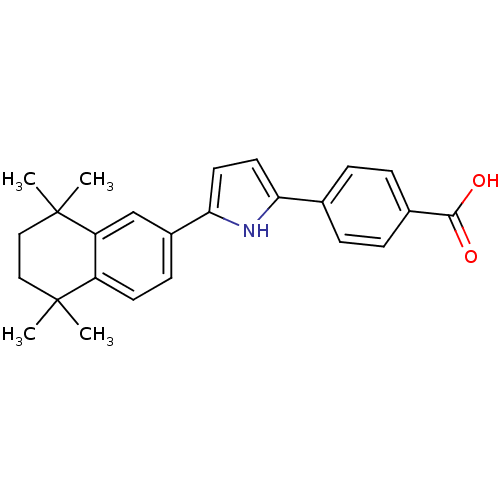
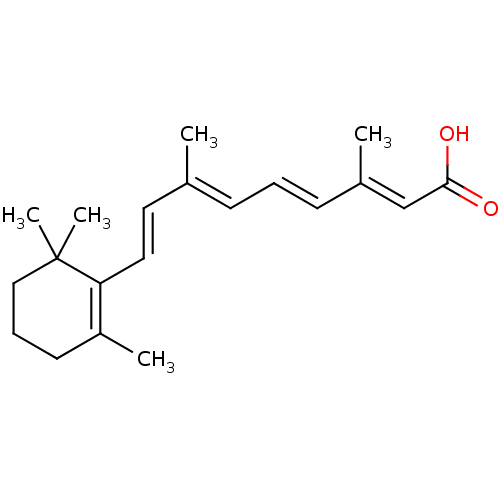

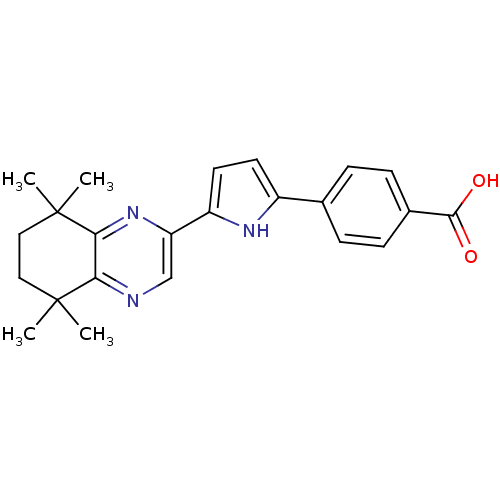
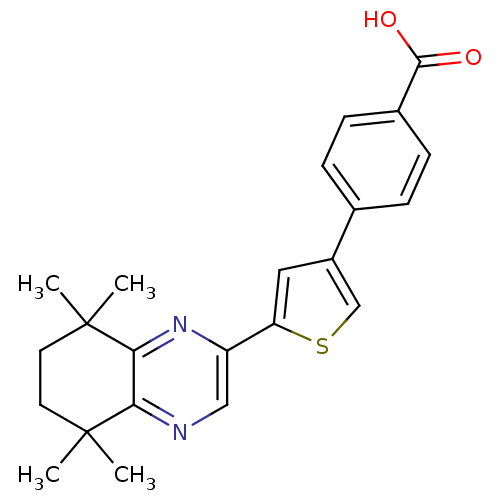
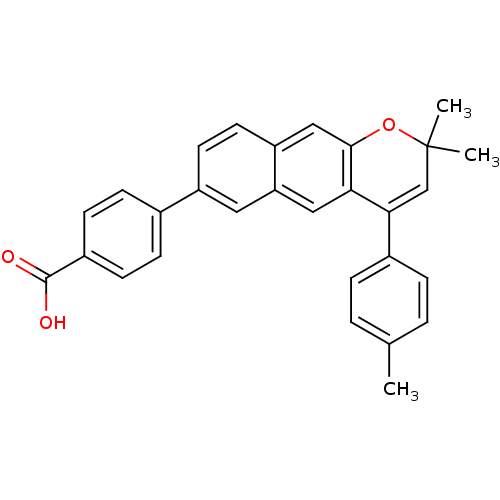
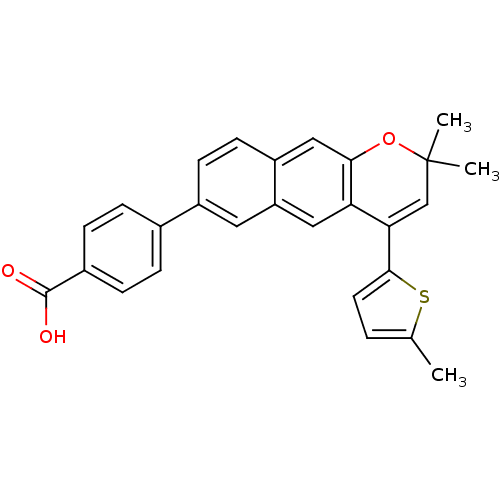
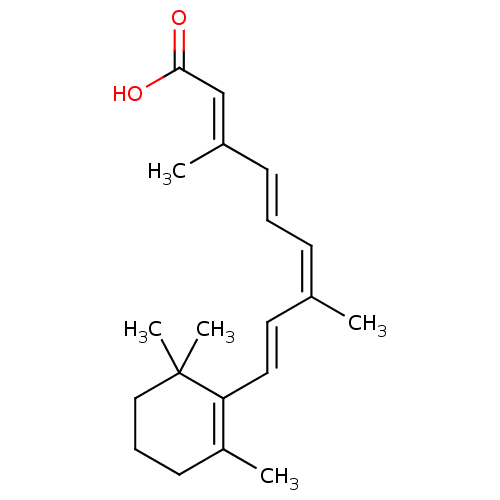
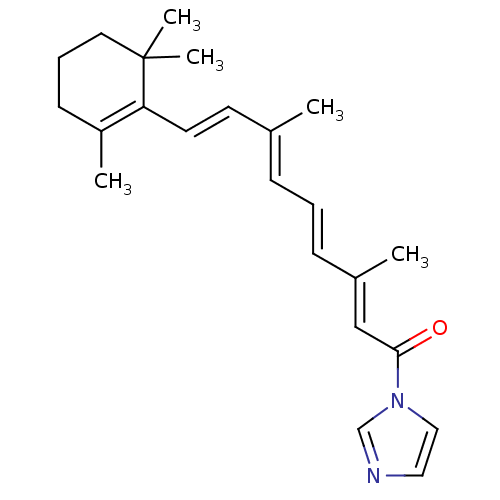
 3D Structure (crystal)
3D Structure (crystal)