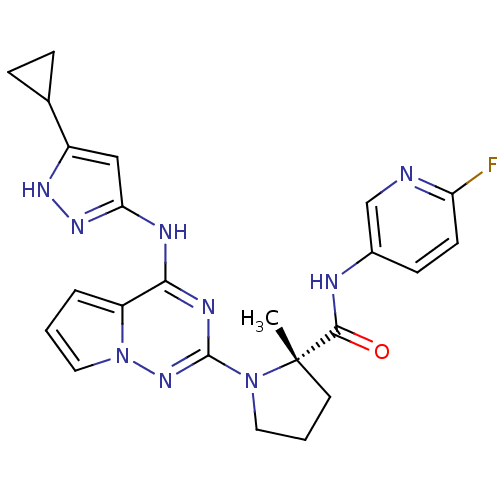
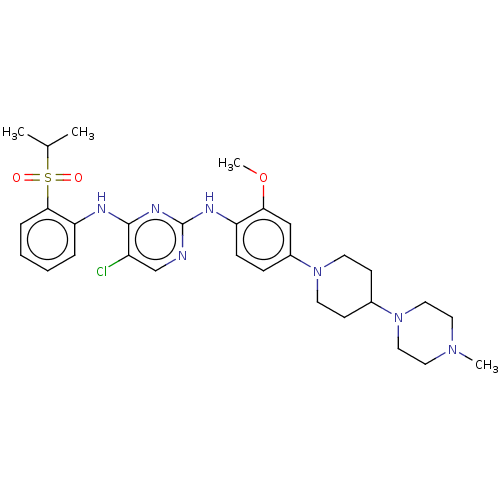
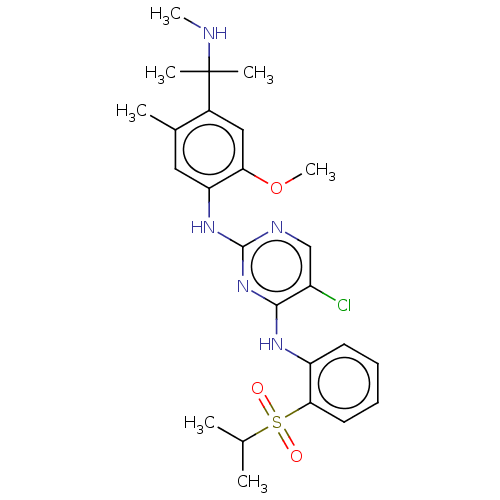
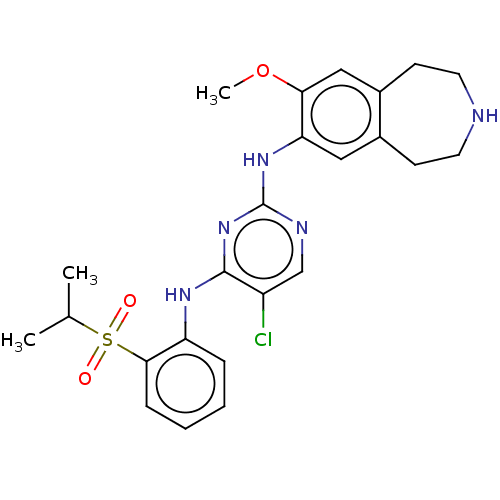
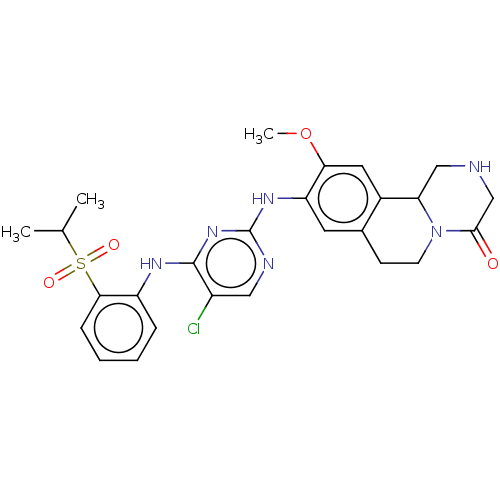
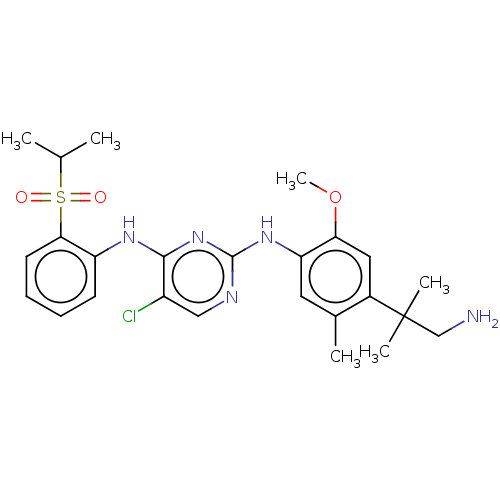
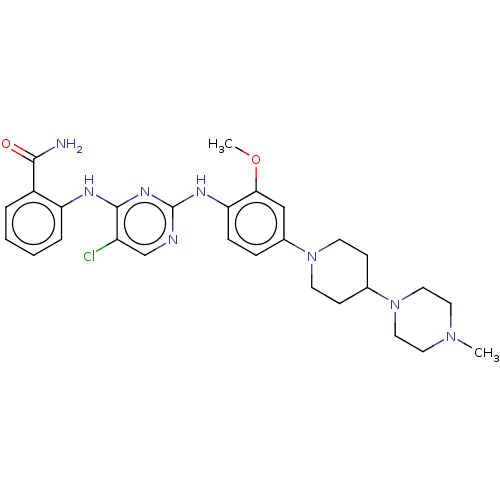
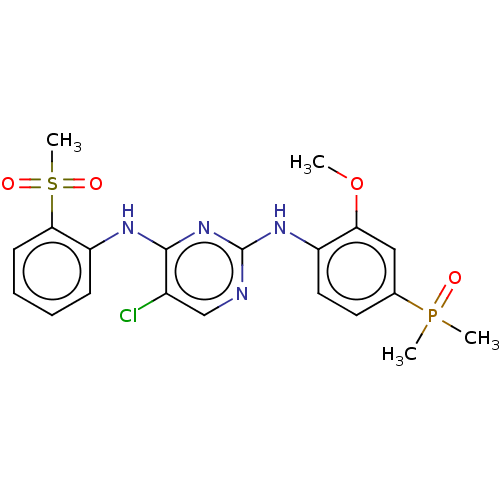
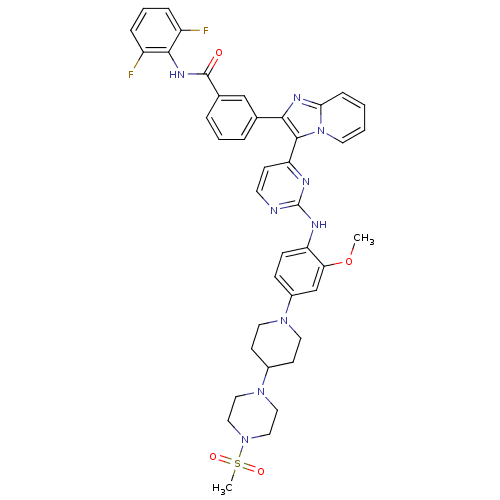
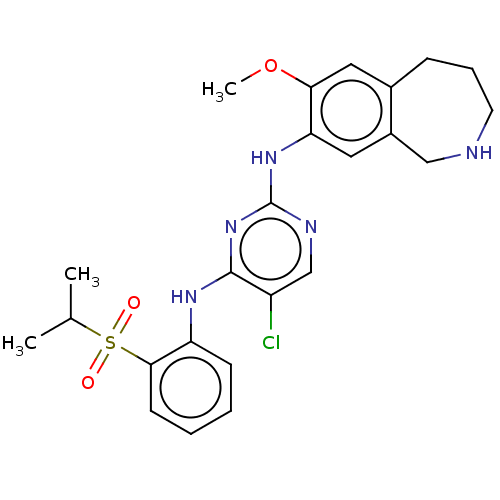
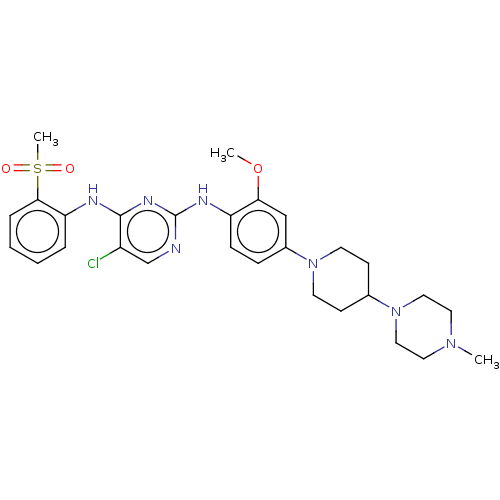
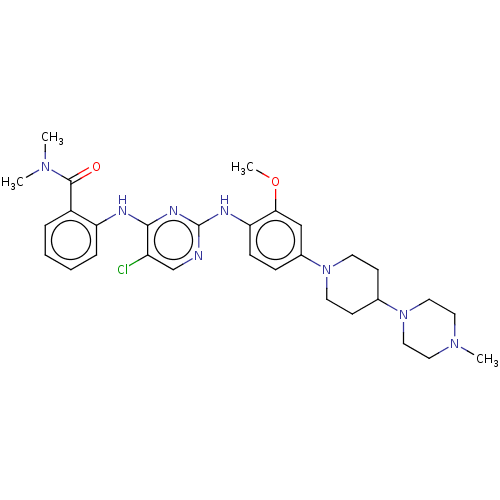
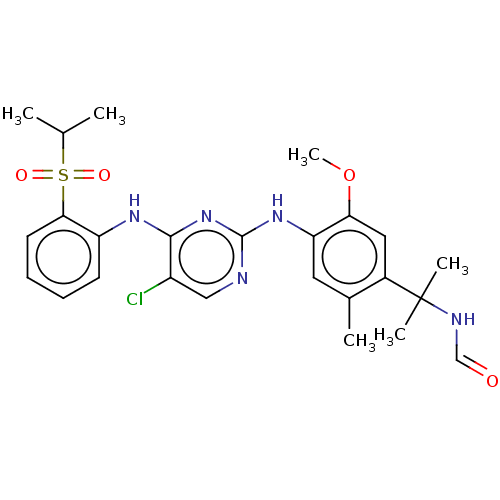
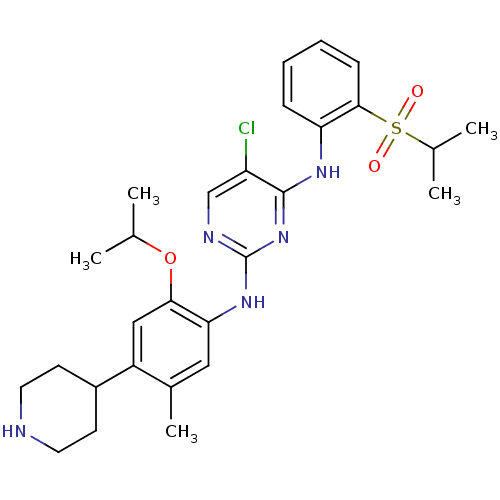
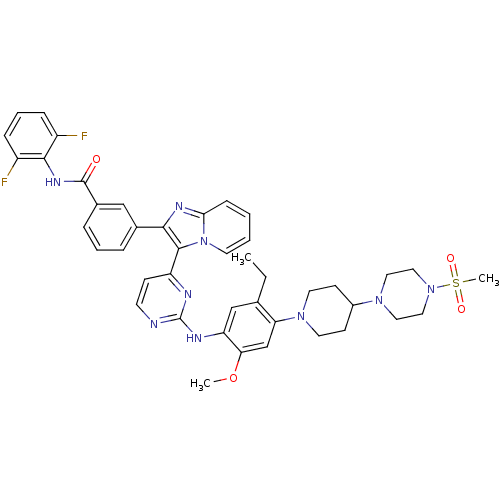
Affinity DataIC50: 0.880nMAssay Description:Inhibition of human InsR using myelin basic protein as substrate and [gamma-33P]ATP measured after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human InsR expressed in mouse IGF1R knockout MEF cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Inhibition of IR (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Inhibition of human InsR using myelin basic protein as substrate and [gamma-33P]ATP measured after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of insulin receptor (unknown origin) by homogeneous time resolved fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:In vitro ability to inhibit the binding of [3H]spiperone to dopamine receptor D2 in rat striatal membranes.More data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:Inhibition of human InsR using myelin basic protein as substrate and [gamma-33P]ATP measured after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:In vitro ability to inhibit the binding of [3H]spiperone to dopamine receptor D2 in rat striatal membranes.More data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:Inhibition of insulin receptor kinase (unknown origin) using TK as substrate after 30 mins by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Displacement of thioflavin T from insulin receptor by thioflavin-T fluorescent dye assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 3.40nMAssay Description:Inhibition of human InsR using myelin basic protein as substrate and [gamma-33P]ATP measured after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 3.80nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 3.90nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of IR kinaseChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of Tel-fused InsR expressed in mouse BAF3 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70nMAssay Description:Inhibition of human InsR using myelin basic protein as substrate and [gamma-33P]ATP measured after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 4.80nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of GST-tagged insulin receptor expressed in baculovirus by time-resolved fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.10nMAssay Description:Inhibition of insulin receptor (unknown origin) by homogeneous time resolved fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.10nMAssay Description:Inhibition of human InsR using myelin basic protein as substrate and [gamma-33P]ATP measured after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 5.20nMAssay Description:Displacement of thioflavin T from insulin receptor by thioflavin-T fluorescent dye assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.20nMAssay Description:Inhibition of insulin receptor (unknown origin) by homogeneous time resolved fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.70nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of insulin receptor kinase (unknown origin) using TK as substrate after 30 mins by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Displacement of thioflavin T from insulin receptor by thioflavin-T fluorescent dye assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Displacement of thioflavin T from insulin receptor by thioflavin-T fluorescent dye assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6.10nMAssay Description:Inhibition of human InsR using myelin basic protein as substrate and [gamma-33P]ATP measured after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 6.10nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of insulin receptor (unknown origin) after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of GST-tagged insulin receptor expressed in baculovirus by time-resolved fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7.5nMAssay Description:Displacement of thioflavin T from insulin receptor by thioflavin-T fluorescent dye assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7.5nMAssay Description:Inhibition of insulin receptor kinase (unknown origin) using TK as substrate after 30 mins by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of IR kinaseChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of recombinant His-tagged human InsR (1011 to 1382 residues) expressed in baculovirus expression system by Z-LYTE assayMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Displacement of thioflavin T from insulin receptor by thioflavin-T fluorescent dye assayMore data for this Ligand-Target Pair