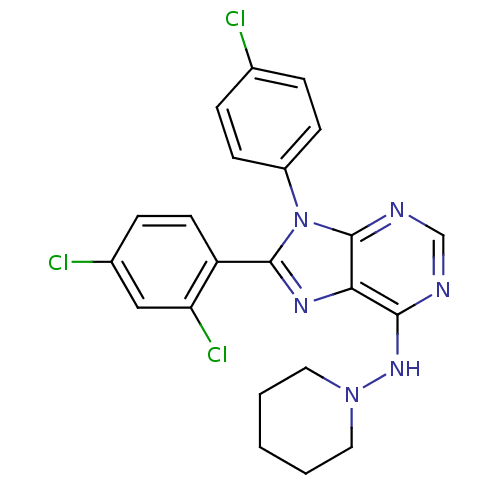
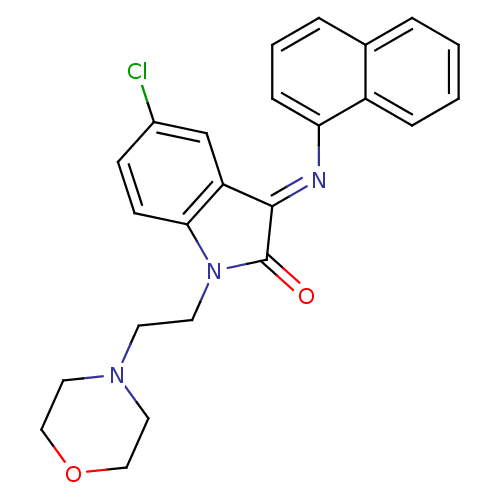
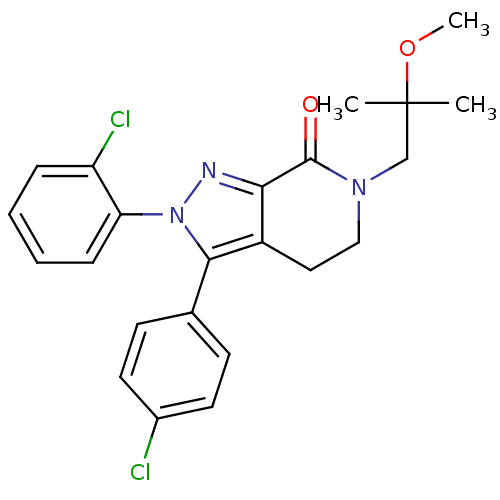
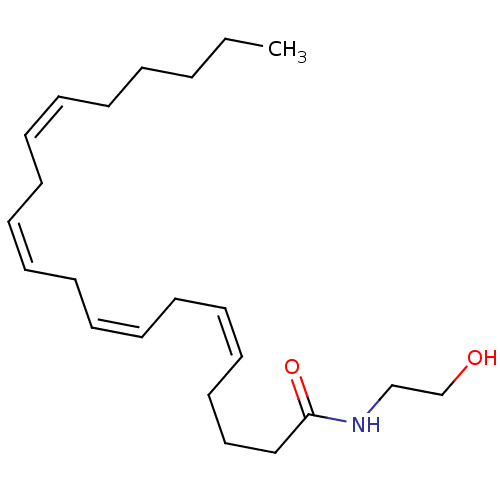
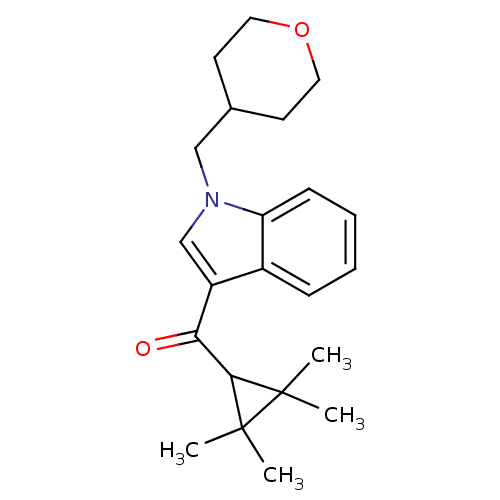
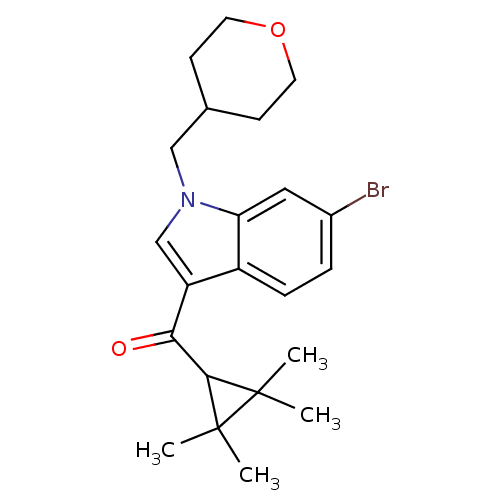
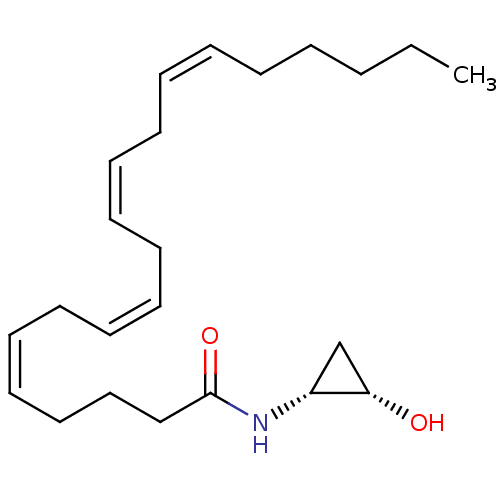
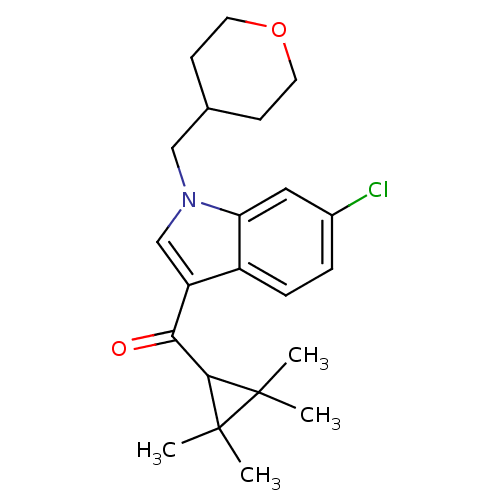
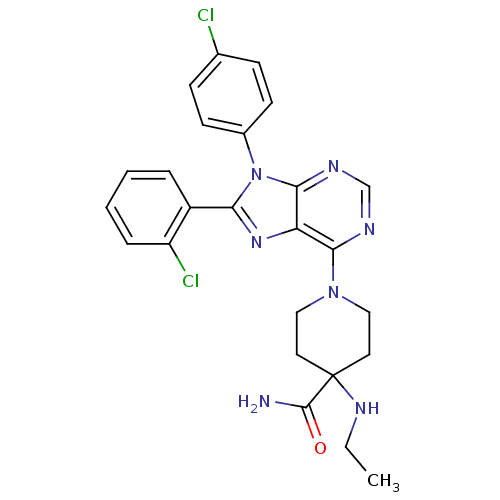
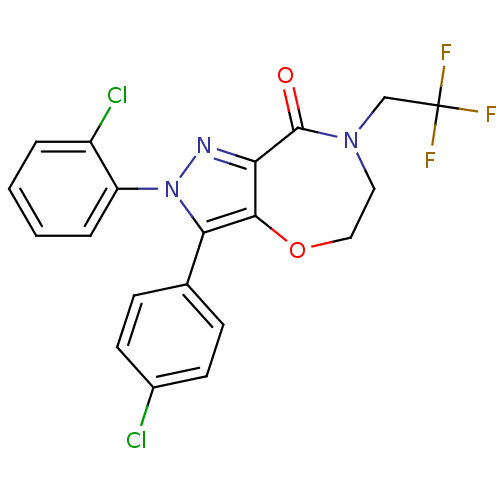
Affinity DataKi: 0.200nM ΔG°: -54.8kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
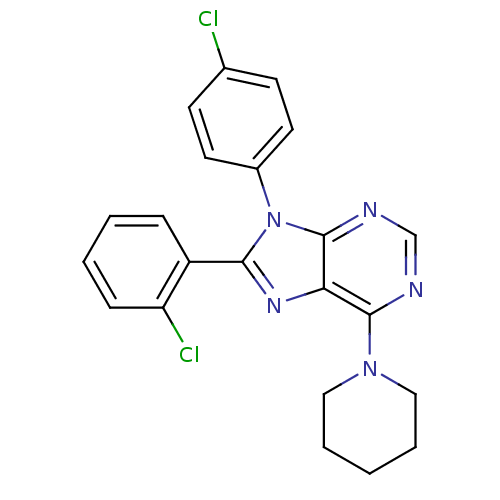
Affinity DataKi: 0.200nM ΔG°: -54.8kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
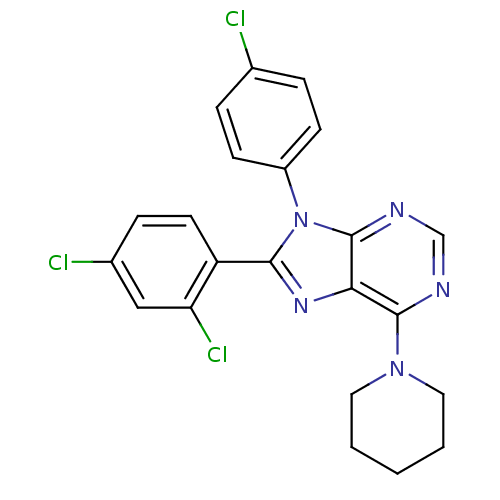
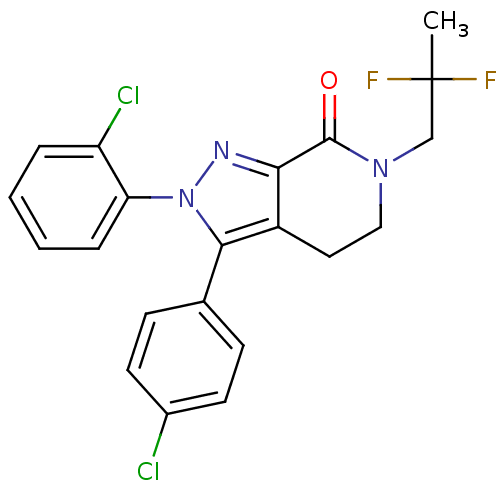
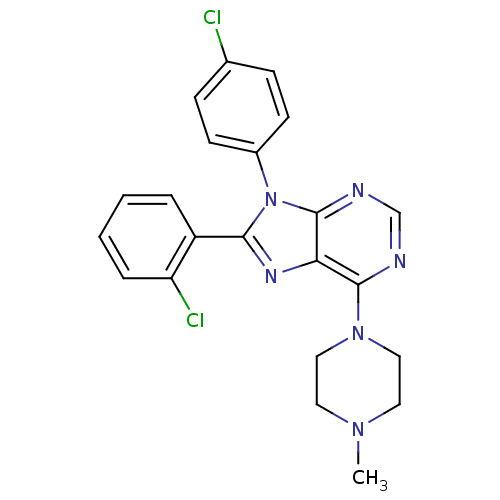
Affinity DataKi: 0.320nM ΔG°: -53.6kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
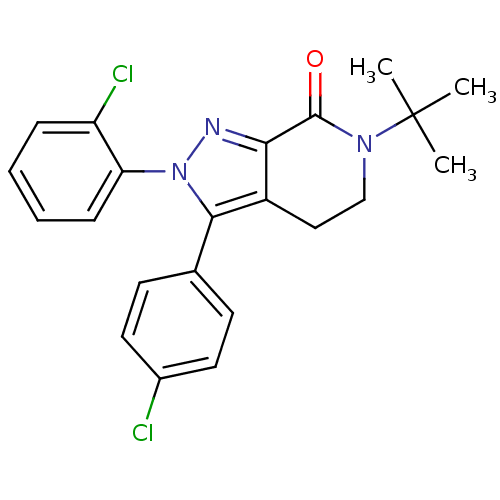
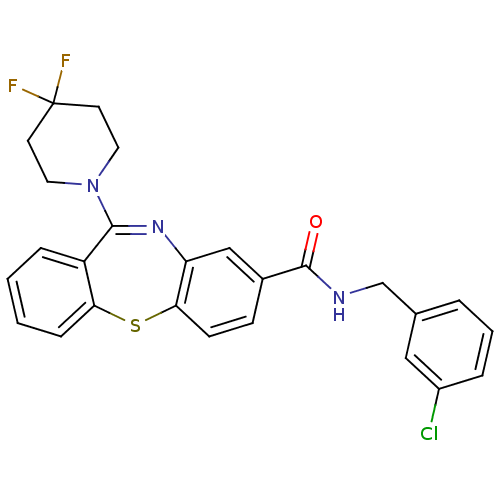
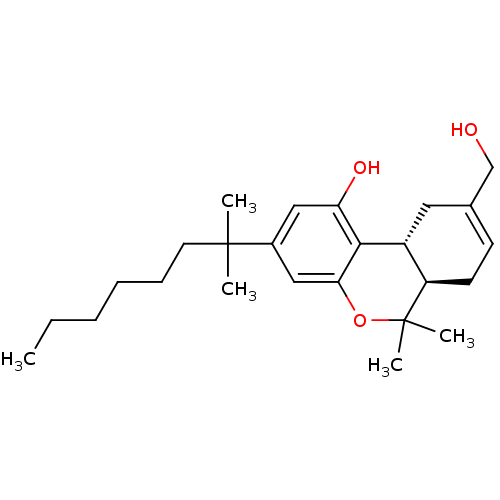
Affinity DataKi: 0.600nM ΔG°: -53.5kJ/mole EC50: 1nMpH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
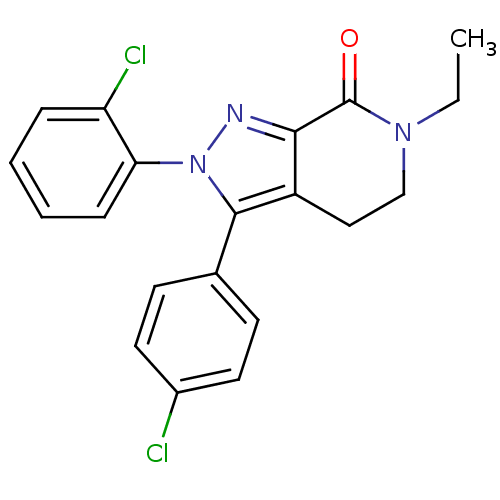
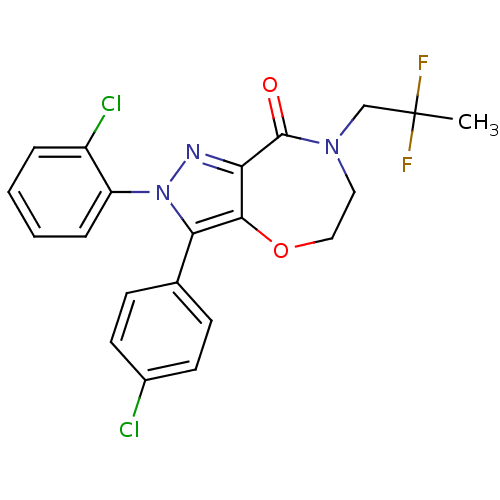
Affinity DataKi: 0.650nM ΔG°: -53.3kJ/molepH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 0.700nM ΔG°: -53.1kJ/molepH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 0.700nM ΔG°: -53.1kJ/mole EC50: 0.800nMpH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 0.790nM ΔG°: -51.4kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DataKi: 0.900nM ΔG°: -52.5kJ/molepH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 1nM ΔG°: -52.2kJ/mole EC50: 0.820nMpH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 1.20nM ΔG°: -50.4kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DataKi: 1.30nM ΔG°: -51.6kJ/mole EC50: 4.80nMpH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 1.40nM ΔG°: -51.4kJ/mole EC50: 0.700nMpH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 1.60nM ΔG°: -49.7kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DataKi: 1.70nM ΔG°: -50.9kJ/mole EC50: 3.10nMpH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 1.80nM ΔG°: -50.7kJ/mole EC50: 1.60nMpH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 1.90nM ΔG°: -50.6kJ/mole EC50: 1.5nMpH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 2nM ΔG°: -49.2kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DataKi: 2.10nM ΔG°: -50.4kJ/molepH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 2.30nM ΔG°: -50.1kJ/molepH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 2.5nM ΔG°: -49.9kJ/mole EC50: 5.5nMpH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 2.5nM ΔG°: -49.9kJ/mole EC50: 9.80nMpH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 2.80nM ΔG°: -49.6kJ/molepH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 2.80nM ΔG°: -49.6kJ/molepH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 2.94nM ΔG°: -49.5kJ/molepH: 7.4 T: 2°CAssay Description:The Kd of CP 55,940 in the isolated CB1 and CB2 receptor expressing membranes was previously determined to be 2.3 nM and 1.5 nM, respectively (see Pe...More data for this Ligand-Target Pair
Affinity DataKi: 3nM ΔG°: -48.2kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DataKi: 3nM ΔG°: -48.2kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DataKi: 3.40nMpH: 7.4Assay Description:Cell membrane homogenates (25 μg protein) were incubated for 120 min at 37° C. with 0.5 nM [3H]CP 55940 (the reference standard [Rinaldi-Carmona...More data for this Ligand-Target Pair
Affinity DataKi: 3.60nM ΔG°: -49.0kJ/molepH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 4.20nM ΔG°: -48.6kJ/molepH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 4.70nM ΔG°: -48.3kJ/mole EC50: 11nMpH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 4.70nM ΔG°: -48.3kJ/mole EC50: 25nMpH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 5.20nM ΔG°: -48.1kJ/molepH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 5.60nM ΔG°: -47.9kJ/molepH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 5.90nM ΔG°: -47.8kJ/molepH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 6.10nM ΔG°: -47.7kJ/molepH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 7.10nM ΔG°: -47.3kJ/molepH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 7.30nM ΔG°: -46.4kJ/molepH: 7.4 T: 2°CAssay Description:CB1 competitive assay were performed in rat cerebellar membranes using [3H]WIN 55212-2 from Perkin-Elmer as a radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 7.40nM ΔG°: -47.2kJ/molepH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 7.5nM ΔG°: -47.2kJ/molepH: 7.4 T: 2°CAssay Description:In this assay, 18 ug of crude membrane expressing hCB1 or hCB2 receptors were re-suspended in 300 uL of binding buffer containing 50 mM Tris-HCl, 2.5...More data for this Ligand-Target Pair
Affinity DataKi: 8.40nM ΔG°: -46.1kJ/molepH: 7.4 T: 2°CAssay Description:CB1 competitive assay were performed in rat cerebellar membranes using [3H]WIN 55212-2 from Perkin-Elmer as a radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 11nM ΔG°: -46.2kJ/mole EC50: 13nMpH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 11nM ΔG°: -46.2kJ/molepH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DataKi: 11nM ΔG°: -46.2kJ/molepH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DataKi: 12nM ΔG°: -46.0kJ/molepH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 12nM ΔG°: -46.0kJ/molepH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 13nM ΔG°: -45.8kJ/molepH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 13.3nM ΔG°: -45.7kJ/molepH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair
Affinity DataKi: 14nM ΔG°: -45.6kJ/molepH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DataKi: 14nM ΔG°: -45.6kJ/molepH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c...More data for this Ligand-Target Pair

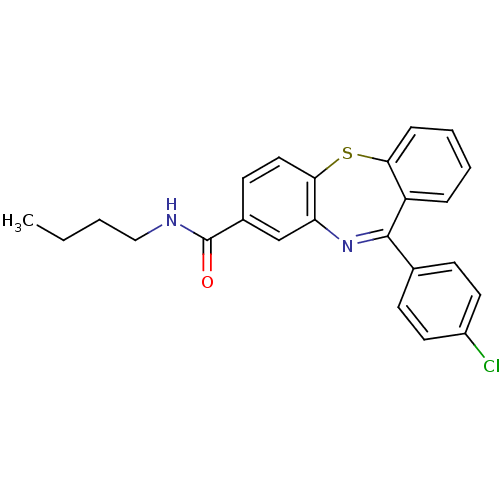
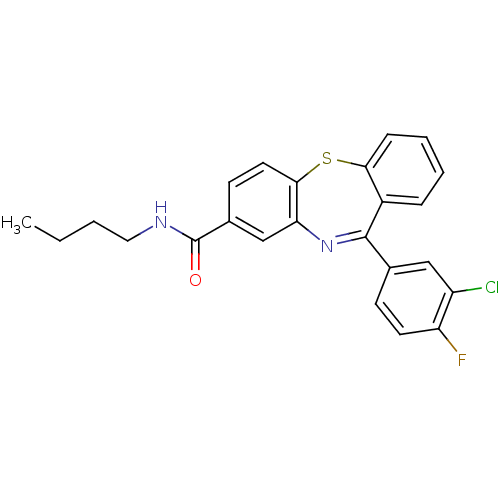
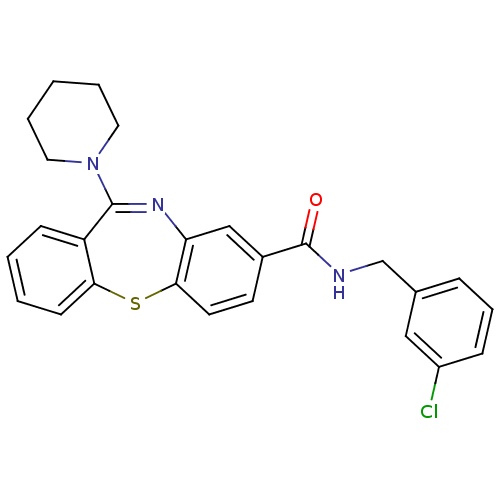
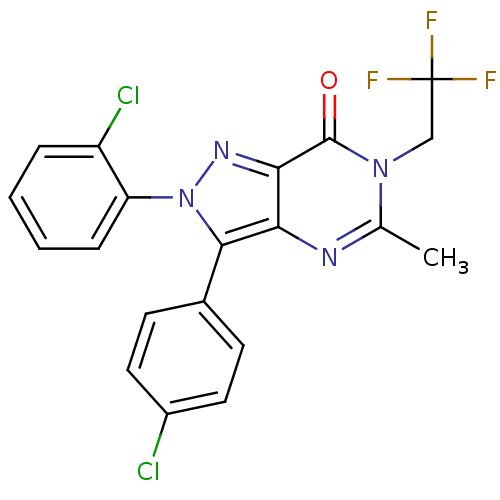
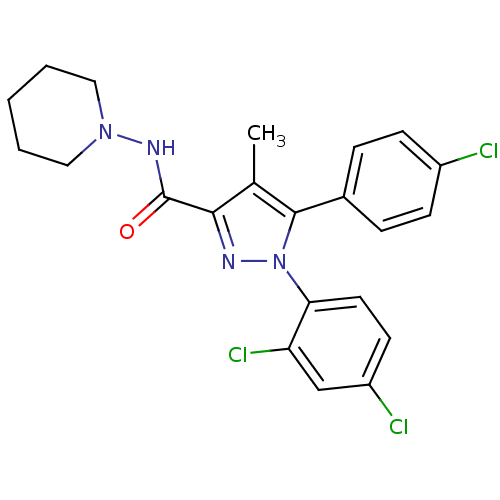
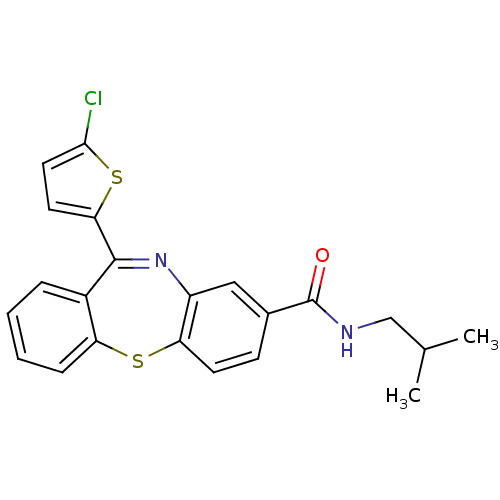

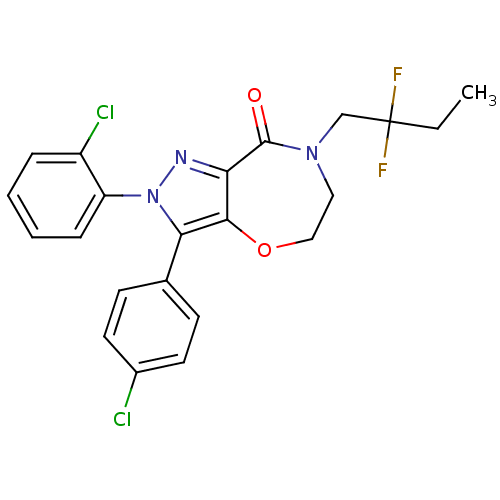
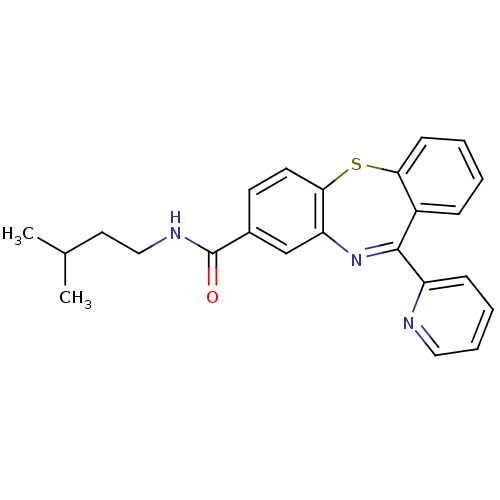
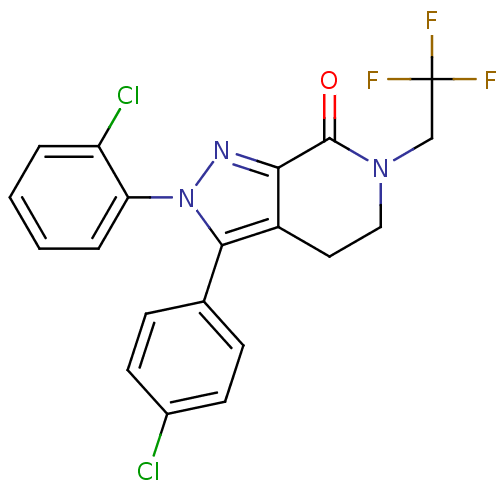


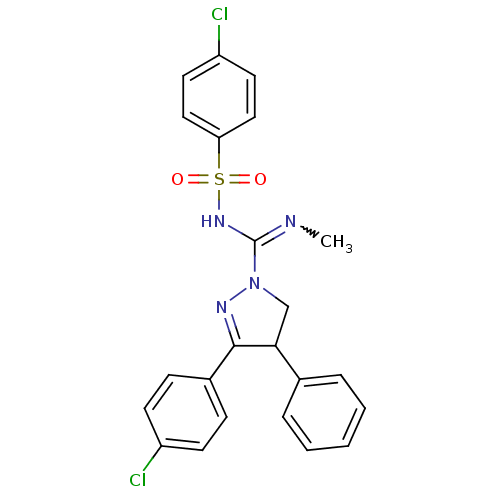

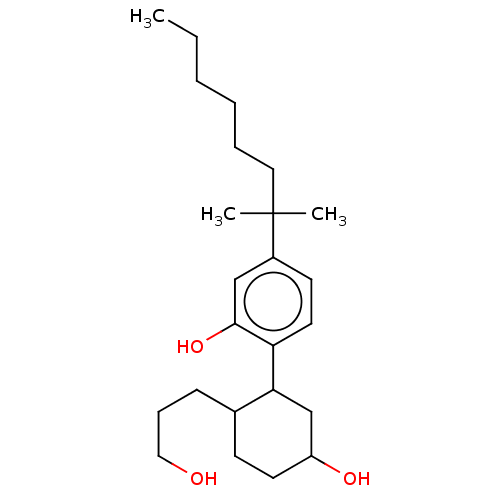
 3D Structure (crystal)
3D Structure (crystal)