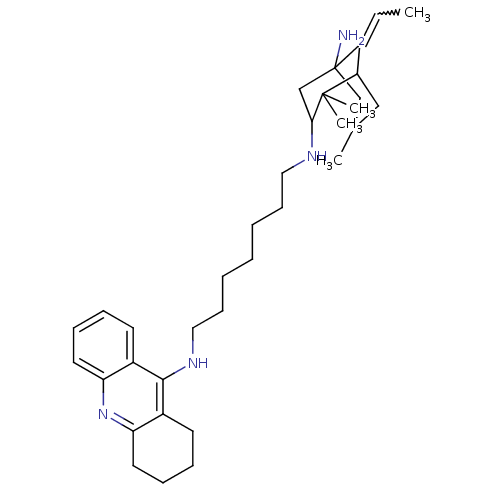
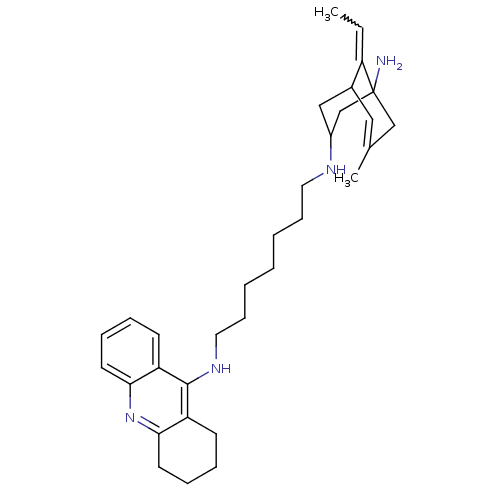
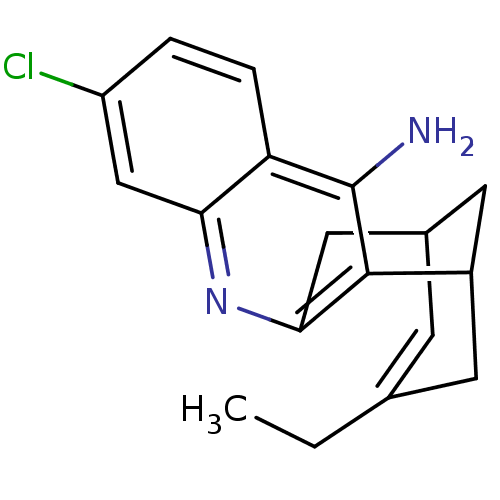
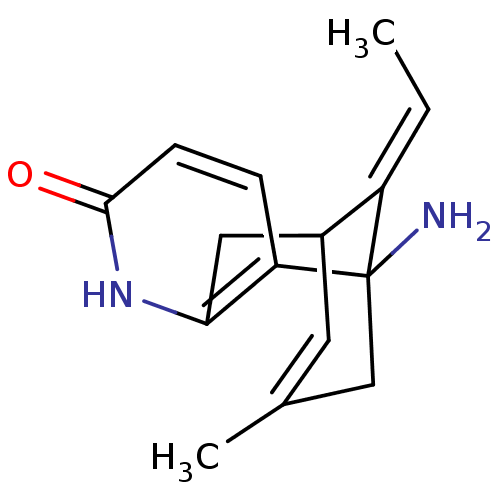
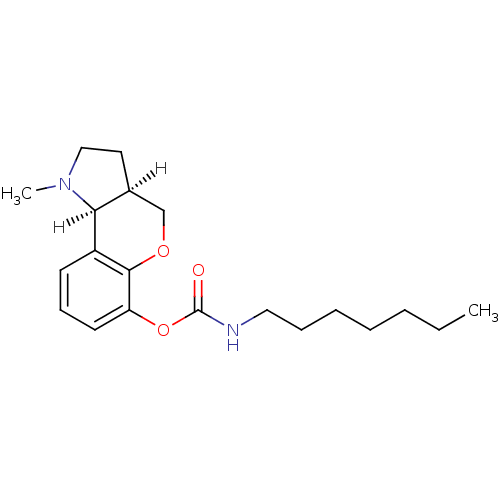
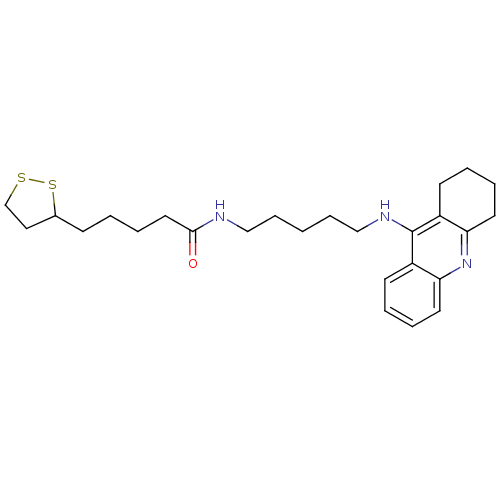
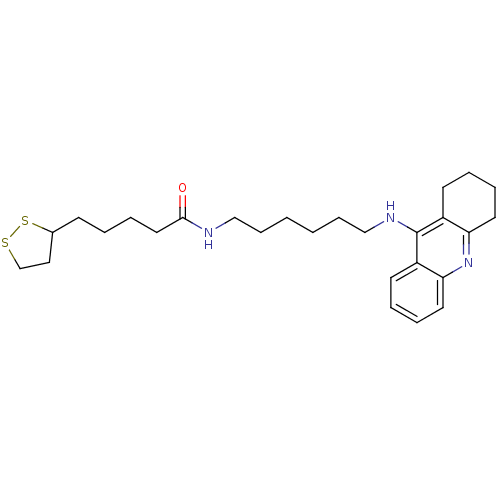
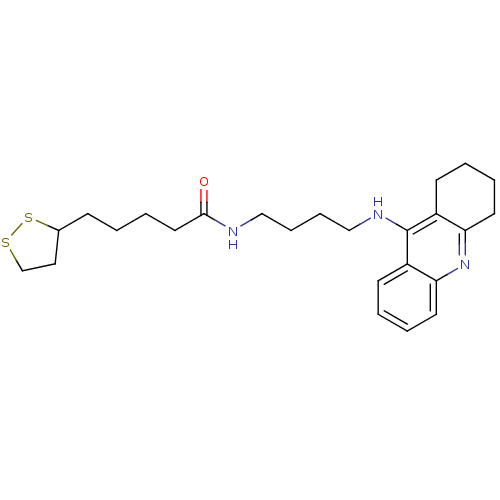
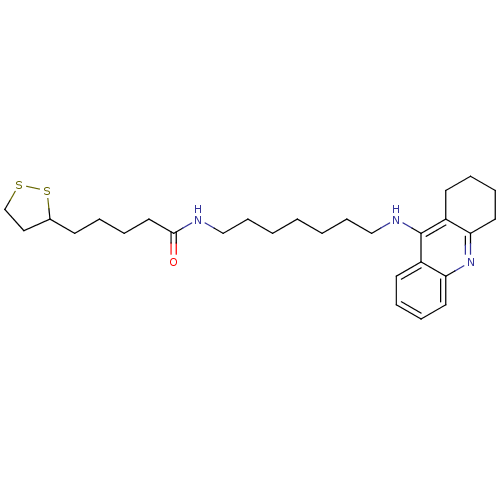
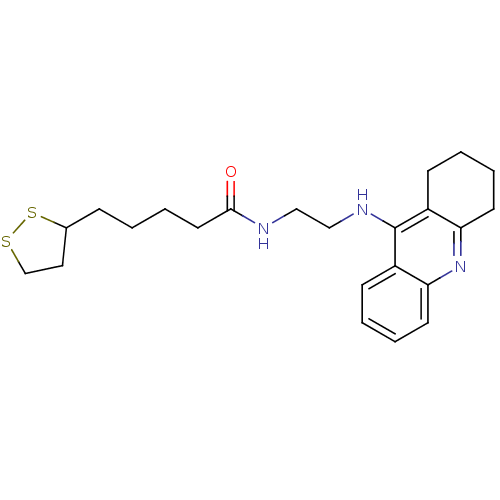
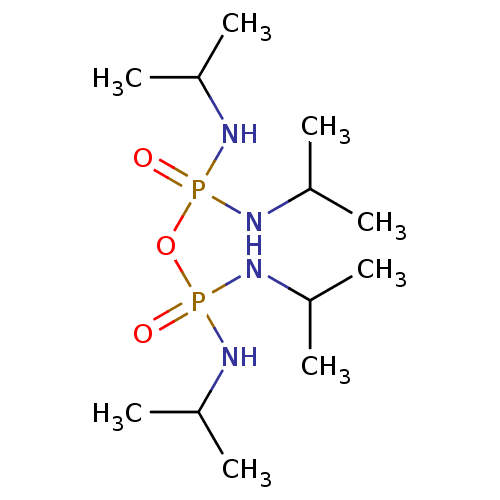
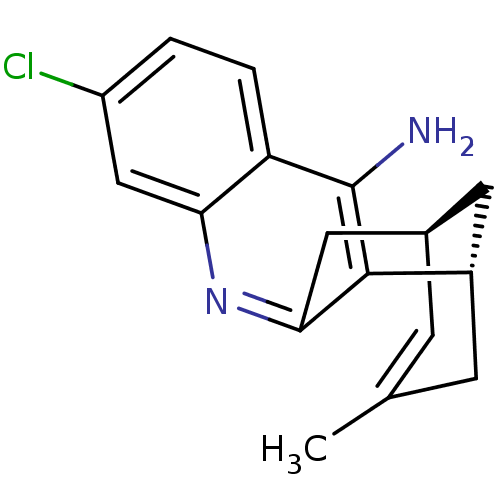
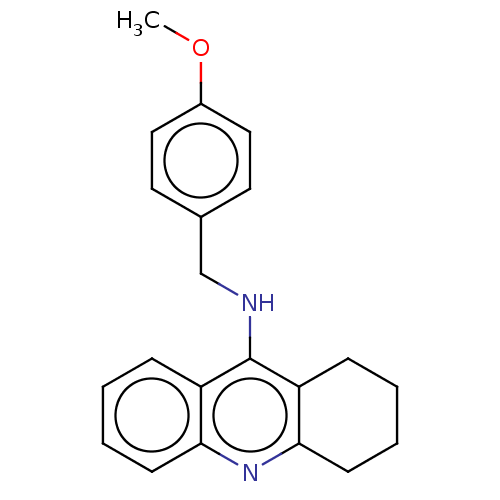
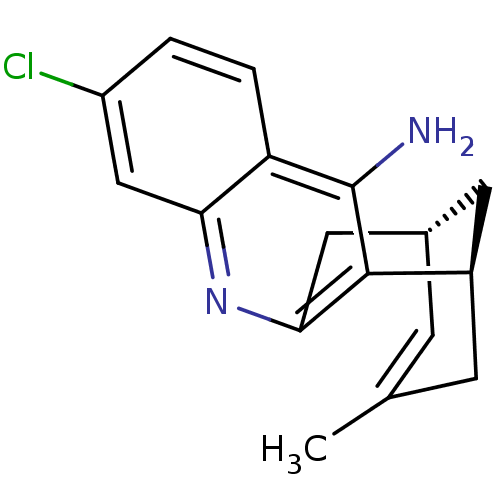
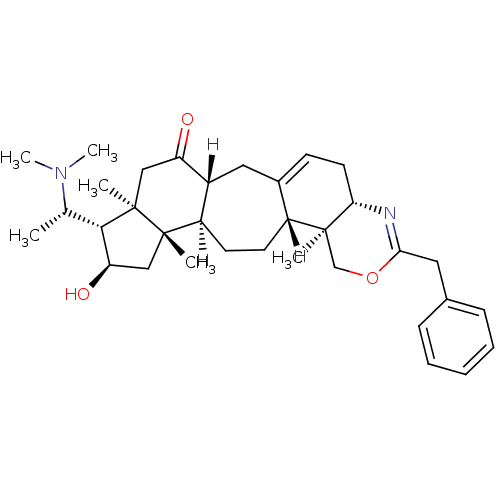
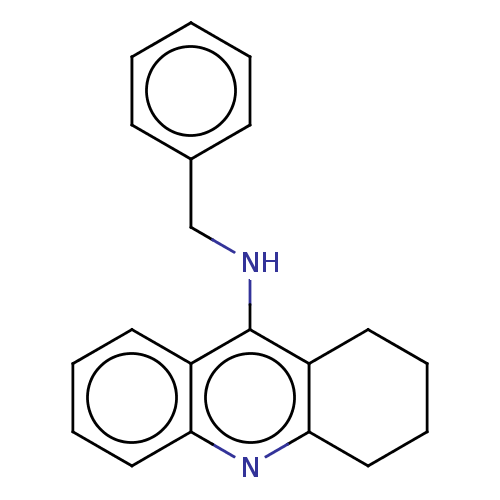
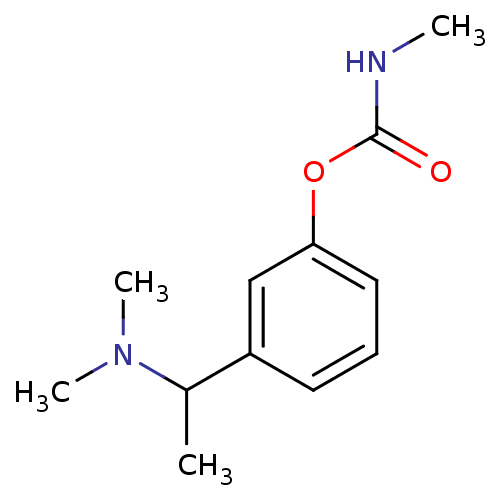
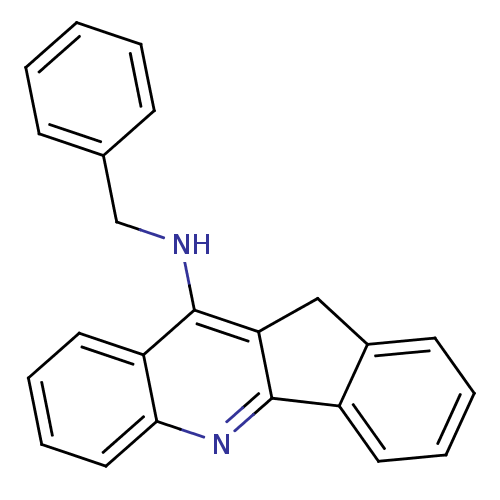
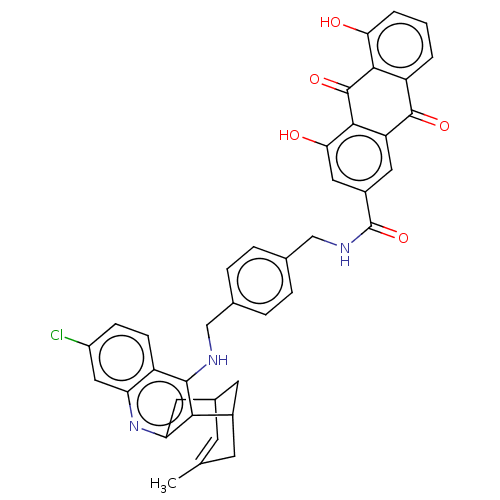
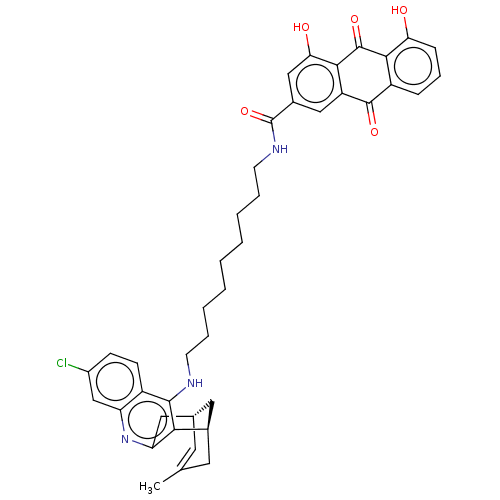
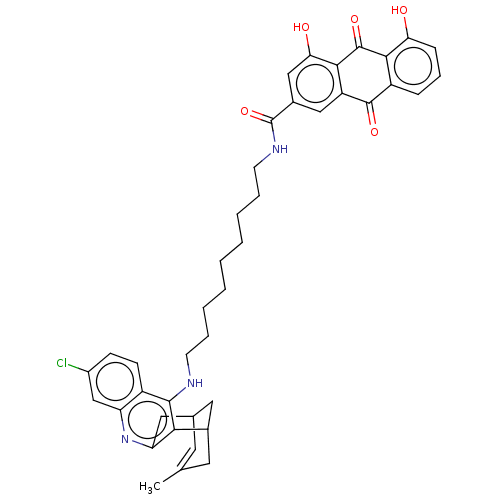
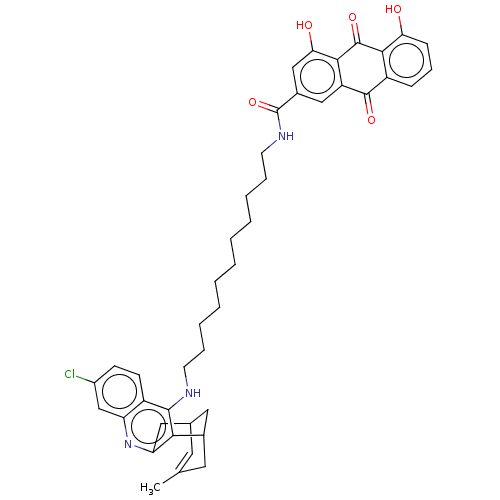
Affinity DataKi: 7nM ΔG°: -46.5kJ/molepH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c...More data for this Ligand-Target Pair
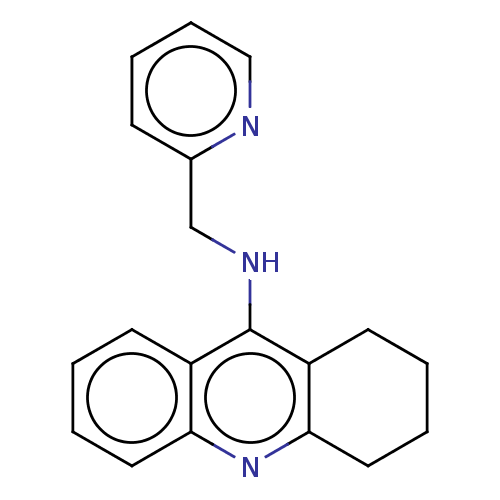
Affinity DataKi: 19.5nM ΔG°: -44.0kJ/molepH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c...More data for this Ligand-Target Pair
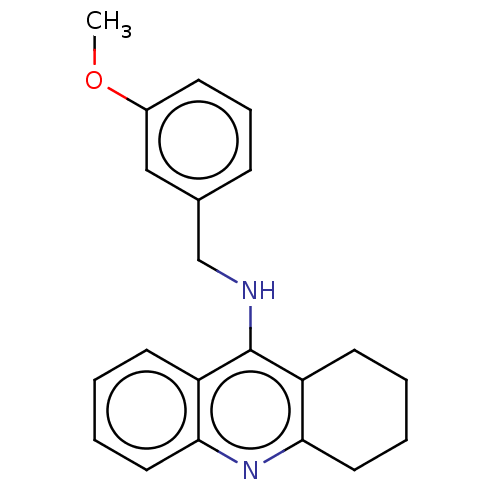
Affinity DataKi: 20.8nM ΔG°: -43.8kJ/molepH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c...More data for this Ligand-Target Pair
Affinity DataKi: 30.8nM ΔG°: -42.9kJ/molepH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c...More data for this Ligand-Target Pair
Affinity DataKi: 120nM ΔG°: -39.5kJ/molepH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nM ΔG°: >-34.2kJ/molepH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c...More data for this Ligand-Target Pair
Affinity DataIC50: 1.26nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at...More data for this Ligand-Target Pair
Affinity DataIC50: 1.48nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at...More data for this Ligand-Target Pair
Affinity DataIC50: 3.24nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at...More data for this Ligand-Target Pair
Affinity DataIC50: 5.04nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at...More data for this Ligand-Target Pair
Affinity DataIC50: 8.58nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at...More data for this Ligand-Target Pair
Affinity DataIC50: 10.5nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at...More data for this Ligand-Target Pair
Affinity DataIC50: 10.8nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMpH: 8.0 T: 2°CAssay Description:Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc...More data for this Ligand-Target Pair
Affinity DataIC50: 24.5nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at...More data for this Ligand-Target Pair
Affinity DataIC50: 30.3nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at...More data for this Ligand-Target Pair
Affinity DataIC50: 36nMpH: 7.2 T: 2°CAssay Description:The inhibitory activity of the compounds towards BuChE was determined following the method of Ellman using BuChE from human serum and butyrylthiochol...More data for this Ligand-Target Pair
Affinity DataIC50: 40nMpH: 7.7Assay Description:Total volume of the reaction mixture was 100 無 containing 60 無, Na2HPO4 buffer, 50 mM and pH 7.7. Ten 無 test compound 0.5 mM well-1 was added foll...More data for this Ligand-Target Pair
Affinity DataIC50: 40nMpH: 8.0 T: 2°CAssay Description:Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc...More data for this Ligand-Target Pair
Affinity DataIC50: 44.4nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at...More data for this Ligand-Target Pair
Affinity DataIC50: 45.8nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at...More data for this Ligand-Target Pair
Affinity DataIC50: 47.5nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 59.4nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at...More data for this Ligand-Target Pair
Affinity DataIC50: 70nMpH: 8.0 T: 2°CAssay Description:Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc...More data for this Ligand-Target Pair
Affinity DataIC50: 80nMpH: 8.0 T: 2°CAssay Description:Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc...More data for this Ligand-Target Pair
Affinity DataIC50: 80nMpH: 8.0 T: 2°CAssay Description:Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc...More data for this Ligand-Target Pair
Affinity DataIC50: 90nMpH: 8.0 T: 2°CAssay Description:Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc...More data for this Ligand-Target Pair
Affinity DataIC50: 134nMpH: 8.0Assay Description:The reaction mixture was consisted of 100 無 of the total volume. To the solution of PBS (pH 8.0, 30 無) consisted of KH2PO4 and K2HPO4 in 96-well pl...More data for this Ligand-Target Pair
Affinity DataIC50: 170nMpH: 8.0 T: 2°CAssay Description:BChE inhibitory activity determinations were carried out similarly by
the method of Ellman et al., using 0.02 unit/mL of human serum BChE and
300 u...More data for this Ligand-Target Pair
Affinity DataIC50: 181nMpH: 8.0 T: 2°CAssay Description:BChE inhibitory activity determinations were carried out similarly by
the method of Ellman et al., using 0.02 unit/mL of human serum BChE and
300 u...More data for this Ligand-Target Pair
Affinity DataIC50: 200nMpH: 8.0 T: 2°CAssay Description:Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc...More data for this Ligand-Target Pair
Affinity DataIC50: 200nMpH: 8.0 T: 2°CAssay Description:Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc...More data for this Ligand-Target Pair
Affinity DataIC50: 209nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at...More data for this Ligand-Target Pair
Affinity DataIC50: 222nMpH: 8.0 T: 2°CAssay Description:BChE inhibitory activity determinations were carried out similarly by
the method of Ellman et al., using 0.02 unit/mL of human serum BChE and
300 u...More data for this Ligand-Target Pair
Affinity DataIC50: 265nMpH: 8.0 T: 2°CAssay Description:BChE inhibitory activity determinations were carried out similarly by
the method of Ellman et al., using 0.02 unit/mL of human serum BChE and
300 u...More data for this Ligand-Target Pair
Affinity DataIC50: 301nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at...More data for this Ligand-Target Pair
Affinity DataIC50: 329nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at...More data for this Ligand-Target Pair
Affinity DataIC50: 331nMpH: 8.0 T: 2°CAssay Description:BChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using BChE from human serum and butyrylthiocholine as substrat...More data for this Ligand-Target Pair
Affinity DataIC50: 350nMpH: 8.0 T: 2°CAssay Description:BChE inhibitory activity determinations were carried out similarly by
the method of Ellman et al., using 0.02 unit/mL of human serum BChE and
300 u...More data for this Ligand-Target Pair
Affinity DataIC50: 375nMpH: 8.0 T: 2°CAssay Description:Inhibition of BuChE activity was determined by the spectroscopic method of Ellman using butyrylthiocholine iodide as substrate, in 96-well microtiter...More data for this Ligand-Target Pair
Affinity DataIC50: 380nMpH: 8.0 T: 2°CAssay Description:Inhibition of BuChE activity was determined by the spectroscopic method of Ellman using butyrylthiocholine iodide as substrate, in 96-well microtiter...More data for this Ligand-Target Pair
Affinity DataIC50: 400nMpH: 8.0 T: 2°CAssay Description:Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc...More data for this Ligand-Target Pair
Affinity DataIC50: 406nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at...More data for this Ligand-Target Pair
Affinity DataIC50: 500nMpH: 8.0 T: 2°CAssay Description:The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ...More data for this Ligand-Target Pair
Affinity DataIC50: 510nMpH: 8.0 T: 2°CAssay Description:BChE inhibitory activity determinations were carried out similarly by
the method of Ellman et al., using 0.02 unit/mL of human serum BChE and
300 u...More data for this Ligand-Target Pair
Affinity DataIC50: 513nMpH: 8.0 T: 2°CAssay Description:BChE inhibitory activity determinations were carried out similarly by
the method of Ellman et al., using 0.02 unit/mL of human serum BChE and
300 u...More data for this Ligand-Target Pair
Affinity DataIC50: 620nMpH: 8.0 T: 2°CAssay Description:BChE inhibitory activity determinations were carried out similarly by
the method of Ellman et al., using 0.02 unit/mL of human serum BChE and
300 u...More data for this Ligand-Target Pair
Affinity DataIC50: 645nMpH: 8.0 T: 2°CAssay Description:BChE inhibitory activity determinations were carried out similarly by
the method of Ellman et al., using 0.02 unit/mL of human serum BChE and
300 u...More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)