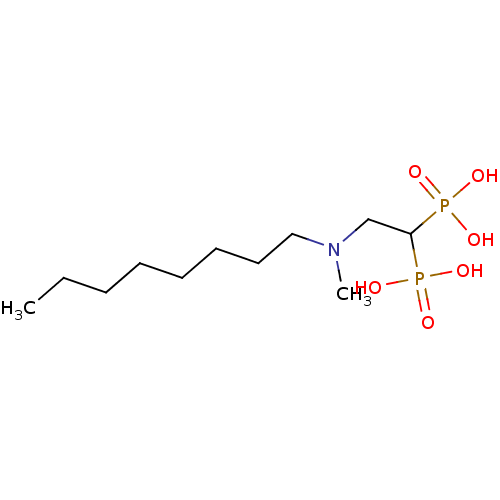
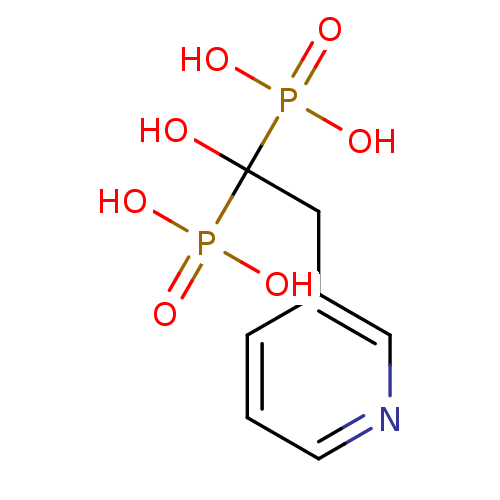
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute Of Biological Chemistry
Curated by ChEMBL
Institute Of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Binding affinity to human GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute Of Biological Chemistry
Curated by ChEMBL
Institute Of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 60nMAssay Description:Binding affinity to human GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute Of Biological Chemistry
Curated by ChEMBL
Institute Of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 70nMAssay Description:Binding affinity to human GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute Of Biological Chemistry
Curated by ChEMBL
Institute Of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 110nMAssay Description:Binding affinity to human GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute Of Biological Chemistry
Curated by ChEMBL
Institute Of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 110nMAssay Description:Binding affinity to human GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute Of Biological Chemistry
Curated by ChEMBL
Institute Of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 230nMAssay Description:Binding affinity to human GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute Of Biological Chemistry
Curated by ChEMBL
Institute Of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 1.80E+3nMAssay Description:Binding affinity to human GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute Of Biological Chemistry
Curated by ChEMBL
Institute Of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 2.70E+3nMAssay Description:Binding affinity to human GGPPSMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute Of Biological Chemistry
Curated by ChEMBL
Institute Of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 3.30E+3nMAssay Description:Inhibition of human GGPPSChecked by AuthorMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute Of Biological Chemistry
Curated by ChEMBL
Institute Of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 6.90E+3nMAssay Description:Inhibition of human GGPPSChecked by AuthorMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute Of Biological Chemistry
Curated by ChEMBL
Institute Of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 1.20E+4nMAssay Description:Inhibition of human GGPPSChecked by AuthorMore data for this Ligand-Target Pair
TargetGeranylgeranyl pyrophosphate synthase(Homo sapiens (Human))
Institute Of Biological Chemistry
Curated by ChEMBL
Institute Of Biological Chemistry
Curated by ChEMBL
Affinity DataKi: 8.30E+6nMAssay Description:Inhibition of human GGPPSChecked by AuthorMore data for this Ligand-Target Pair



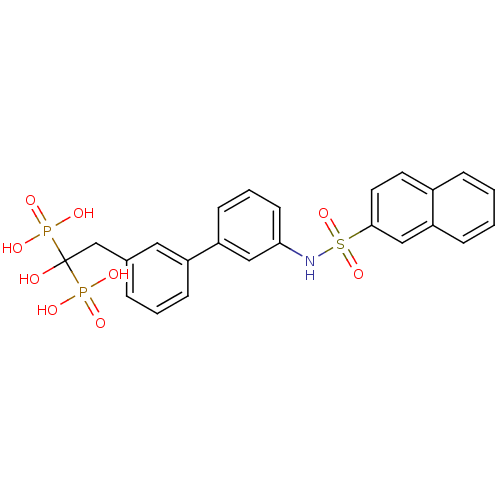


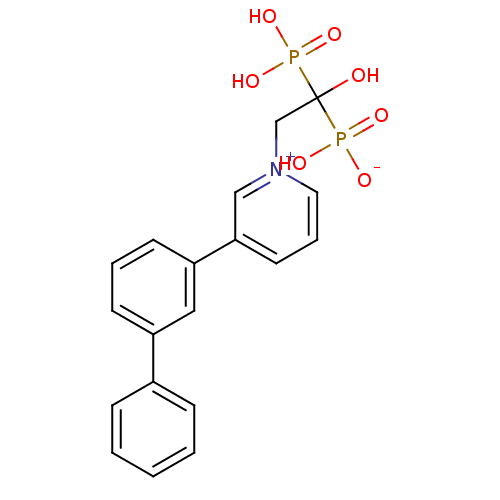
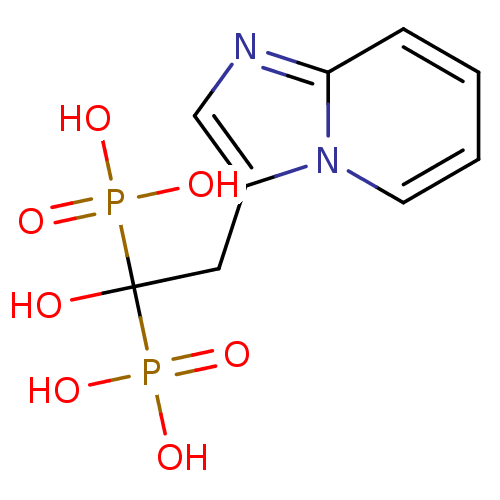
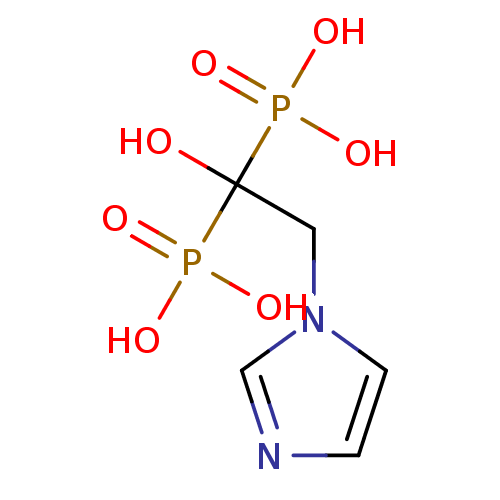
 3D Structure (crystal)
3D Structure (crystal)