Reaction Details  Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataCell Reactant:
HIV-1 Protease Mutant (V82F/I84V)
Syringe Reactant:
BDBM518
Meas. Tech.:
Isothermal Titration Calorimetry
Entry Date.:
04/20/04
ΔG°:
-40.546±n/a (kJ/mole)
pH:
5±n/a
Temperature:
298.15±n/a (K)
ΔH° :
18.392±0.836 (kJ/mole)
ΔHobs :
18.183±n/a (kJ/mole)
Corrected for ΔHioniz:
not known
Protons Released:
0.4
ΔCp :
-1.7138±0.1672 (kJ/mole)
ΔS° :
0.1978±n/a (kJ/mole-K)
Citation
Cell React
Source:
mutations at position 82 and 84 were introduced using an in vitro site-directed mutagenesis kit, mutant protease was expressed in BL21/DE3
Purity:
99%
Prep. Method:
HIV-1 protease was purified and refolded from E. coli inclusion bodies.
Name:
HIV-1 Protease Mutant (V82F/I84V)
Synonyms:
n/a
Type:
Protein Complex
Mol. Mass.:
n/a
Description:
n/a
Components:
This complex has 2 components.
Component 1
Name:
HIV-1 Protease Mutant (V82F/I84V) chain A
Synonyms:
HIV-1 Protease Mutant (V82F/I84V) chain B
Type:
Enzyme Subunit
Mol. Mass.:
10816.23
Organism:
Human immunodeficiency virus type 1
Description:
Using plasmid-encoded mutant protease (Q7K/L33I/L63I designed to remove three hypersensitive autolytic sites) for the stability of protease.
Residue:
99
Sequence:
PQVTLWKRPLVTIKIGGQLKEALLDTGADDTVIEEMSLPGRWKPKMIGGIGGFIKVRQYDQIIIEICGHKAIGTVLVGPTPFNVIGRNLLTQIGCTLNF
Component 2
Name:
HIV-1 Protease Mutant (V82F/I84V) chain A
Synonyms:
HIV-1 Protease Mutant (V82F/I84V) chain B
Type:
Enzyme Subunit
Mol. Mass.:
10816.23
Organism:
Human immunodeficiency virus type 1
Description:
Using plasmid-encoded mutant protease (Q7K/L33I/L63I designed to remove three hypersensitive autolytic sites) for the stability of protease.
Residue:
99
Sequence:
PQVTLWKRPLVTIKIGGQLKEALLDTGADDTVIEEMSLPGRWKPKMIGGIGGFIKVRQYDQIIIEICGHKAIGTVLVGPTPFNVIGRNLLTQIGCTLNF
Syringe React
Source:
Purified from commercial capsules
Prep. Method:
Further purified by HPLC using a semipreparative C-18 reversed-phase column developed with 0-100% acetonitrile in 0.05% TFA
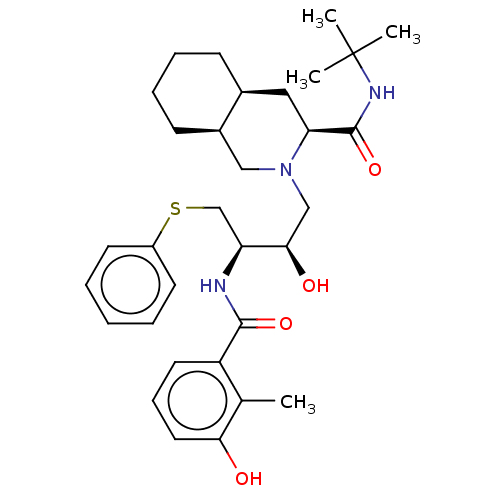
Name:
BDBM518
Synonyms:
(3S,4aS,8aS)-N-tert-butyl-2-[(2R,3R)-2-hydroxy-3-[(3-hydroxy-2-methylphenyl)formamido]-4-(phenylsulfanyl)butyl]-decahydroisoquinoline-3-carboxamide | AG-1343 | CHEMBL584 | Nelfinavir | Viracept
Type:
Small organic molecule
Emp. Form.:
C32H45N3O4S
Mol. Mass.:
567.782
SMILES:
Cc1c(O)cccc1C(=O)N[C@@H](CSc1ccccc1)[C@H](O)CN1C[C@H]2CCCC[C@H]2C[C@H]1C(=O)NC(C)(C)C |@@:23|