Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Atrial natriuretic peptide receptor 1
Ligand
BDBM50112317
Substrate
n/a
Meas. Tech.
ChEBML_144255
EC50
30±n/a nM
Citation
More Info.:
Target
Name:
Atrial natriuretic peptide receptor 1
Synonyms:
ANP-A | ANPRA | ANPRA_HUMAN | Atrial natriuretic peptide A-type receptor | Atrial natriuretic peptide receptor | Atrial natriuretic peptide receptor A | GC-A | Guanylate cyclase | NPR-A | NPR1
Type:
PROTEIN
Mol. Mass.:
118919.35
Organism:
Homo sapiens (Human)
Description:
ChEMBL_700223
Residue:
1061
Sequence:
MPGPRRPAGSRLRLLLLLLLPPLLLLLRGSHAGNLTVAVVLPLANTSYPWSWARVGPAVELALAQVKARPDLLPGWTVRTVLGSSENALGVCSDTAAPLAAVDLKWEHNPAVFLGPGCVYAAAPVGRFTAHWRVPLLTAGAPALGFGVKDEYALTTRAGPSYAKLGDFVAALHRRLGWERQALMLYAYRPGDEEHCFFLVEGLFMRVRDRLNITVDHLEFAEDDLSHYTRLLRTMPRKGRVIYICSSPDAFRTLMLLALEAGLCGEDYVFFHLDIFGQSLQGGQGPAPRRPWERGDGQDVSARQAFQAAKIITYKDPDNPEYLEFLKQLKHLAYEQFNFTMEDGLVNTIPASFHDGLLLYIQAVTETLAHGGTVTDGENITQRMWNRSFQGVTGYLKIDSSGDRETDFSLWDMDPENGAFRVVLNYNGTSQELVAVSGRKLNWPLGYPPPDIPKCGFDNEDPACNQDHLSTLEVLALVGSLSLLGILIVSFFIYRKMQLEKELASELWRVRWEDVEPSSLERHLRSAGSRLTLSGRGSNYGSLLTTEGQFQVFAKTAYYKGNLVAVKRVNRKRIELTRKVLFELKHMRDVQNEHLTRFVGACTDPPNICILTEYCPRGSLQDILENESITLDWMFRYSLTNDIVKGMLFLHNGAICSHGNLKSSNCVVDGRFVLKITDYGLESFRDLDPEQGHTVYAKKLWTAPELLRMASPPVRGSQAGDVYSFGIILQEIALRSGVFHVEGLDLSPKEIIERVTRGEQPPFRPSLALQSHLEELGLLMQRCWAEDPQERPPFQQIRLTLRKFNRENSSNILDNLLSRMEQYANNLEELVEERTQAYLEEKRKAEALLYQILPHSVAEQLKRGETVQAEAFDSVTIYFSDIVGFTALSAESTPMQVVTLLNDLYTCFDAVIDNFDVYKVETIGDAYMVVSGLPVRNGRLHACEVARMALALLDAVRSFRIRHRPQEQLRLRIGIHTGPVCAGVVGLKMPRYCLFGDTVNTASRMESNGEALKIHLSSETKAVLEEFGGFELELRGDVEMKGKGKVRTYWLLGERGSSTRG
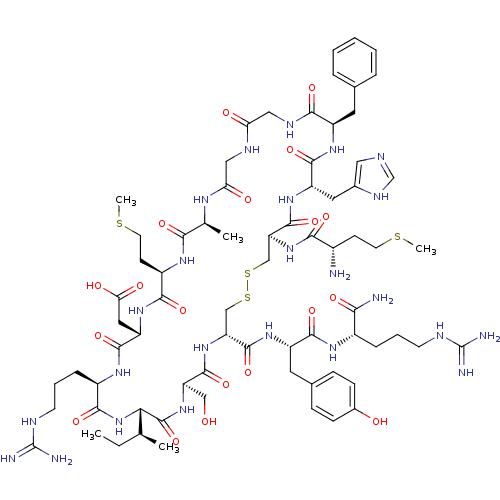
Inhibitor
Name:
BDBM50112317
Synonyms:
Atrial natriuretic peptide analogue | CHEMBL439465 | [Ala7]mintANP
Type:
Small organic molecule
Emp. Form.:
C72H110N24O19S4
Mol. Mass.:
1744.053
SMILES:
CC[C@H](C)[C@@H]1NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](CCSC)NC(=O)[C@H](C)NC(=O)CNC(=O)CNC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CSSC[C@@H](NC(=O)[C@@H](CO)NC1=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O)NC(=O)[C@@H](N)CCSC