Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Heat shock protein HSP 90-beta
Ligand
BDBM15359
Substrate
n/a
Meas. Tech.
ChEMBL_787204 (CHEMBL1918874)
Kd
7.3±n/a nM
Citation
 Vallée, F; Carrez, C; Pilorge, F; Dupuy, A; Parent, A; Bertin, L; Thompson, F; Ferrari, P; Fassy, F; Lamberton, A; Thomas, A; Arrebola, R; Guerif, S; Rohaut, A; Certal, V; Ruxer, JM; Gouyon, T; Delorme, C; Jouanen, A; Dumas, J; Grépin, C; Combeau, C; Goulaouic, H; Dereu, N; Mikol, V; Mailliet, P; Minoux, H Tricyclic series of heat shock protein 90 (Hsp90) inhibitors part I: discovery of tricyclic imidazo[4,5-c]pyridines as potent inhibitors of the Hsp90 molecular chaperone. J Med Chem 54:7206-19 (2011) [PubMed] Article
Vallée, F; Carrez, C; Pilorge, F; Dupuy, A; Parent, A; Bertin, L; Thompson, F; Ferrari, P; Fassy, F; Lamberton, A; Thomas, A; Arrebola, R; Guerif, S; Rohaut, A; Certal, V; Ruxer, JM; Gouyon, T; Delorme, C; Jouanen, A; Dumas, J; Grépin, C; Combeau, C; Goulaouic, H; Dereu, N; Mikol, V; Mailliet, P; Minoux, H Tricyclic series of heat shock protein 90 (Hsp90) inhibitors part I: discovery of tricyclic imidazo[4,5-c]pyridines as potent inhibitors of the Hsp90 molecular chaperone. J Med Chem 54:7206-19 (2011) [PubMed] Article More Info.:
Target
Name:
Heat shock protein HSP 90-beta
Synonyms:
HS90B_HUMAN | HSP 84 | HSP 90 | HSP90AB1 | HSP90B | HSPC2 | HSPCB | Heat Shock Protein 90 (Hsp90) | Heat shock protein HSP 90 (Hsp90)
Type:
Molecular Chaperone
Mol. Mass.:
83229.45
Organism:
Homo sapiens (Human)
Description:
P08238
Residue:
724
Sequence:
MPEEVHHGEEEVETFAFQAEIAQLMSLIINTFYSNKEIFLRELISNASDALDKIRYESLTDPSKLDSGKELKIDIIPNPQERTLTLVDTGIGMTKADLINNLGTIAKSGTKAFMEALQAGADISMIGQFGVGFYSAYLVAEKVVVITKHNDDEQYAWESSAGGSFTVRADHGEPIGRGTKVILHLKEDQTEYLEERRVKEVVKKHSQFIGYPITLYLEKEREKEISDDEAEEEKGEKEEEDKDDEEKPKIEDVGSDEEDDSGKDKKKKTKKIKEKYIDQEELNKTKPIWTRNPDDITQEEYGEFYKSLTNDWEDHLAVKHFSVEGQLEFRALLFIPRRAPFDLFENKKKKNNIKLYVRRVFIMDSCDELIPEYLNFIRGVVDSEDLPLNISREMLQQSKILKVIRKNIVKKCLELFSELAEDKENYKKFYEAFSKNLKLGIHEDSTNRRRLSELLRYHTSQSGDEMTSLSEYVSRMKETQKSIYYITGESKEQVANSAFVERVRKRGFEVVYMTEPIDEYCVQQLKEFDGKSLVSVTKEGLELPEDEEEKKKMEESKAKFENLCKLMKEILDKKVEKVTISNRLVSSPCCIVTSTYGWTANMERIMKAQALRDNSTMGYMMAKKHLEINPDHPIVETLRQKAEADKNDKAVKDLVVLLFETALLSSGFSLEDPQTHSNRIYRMIKLGLGIDEDEVAAEEPNAAVPDEIPPLEGDEDASRMEEVD
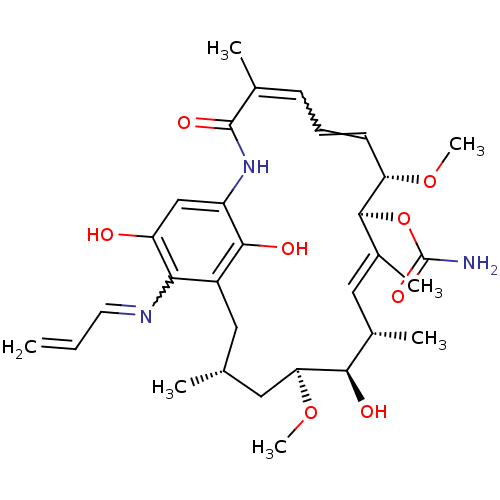
Inhibitor
Name:
BDBM15359
Synonyms:
(4E,6Z,8S,9S,10E,12S,13R,14S,16R)-13-hydroxy-8,14-dimethoxy-4,10,12,16-tetramethyl-3,20,22-trioxo-19-(prop-2-en-1-ylamino)-2-azabicyclo[16.3.1]docosa-1(21),4,6,10,18-pentaen-9-yl carbamate | 17-(Allylamino)geldanamycin | 17-AAG | 17AAG | CHEMBL109480 | GLD-36 | Tanespimycin
Type:
Small organic molecule
Emp. Form.:
C31H43N3O8
Mol. Mass.:
585.6884
SMILES:
CO[C@H]1C[C@H](C)Cc2c(O)c(NC(=O)C(C)=CC=C[C@H](OC)[C@@H](OC(N)=O)\C(C)=C\[C@H](C)[C@H]1O)cc(O)c2N=CC=C |r,w:16.16,38.39,t:28|