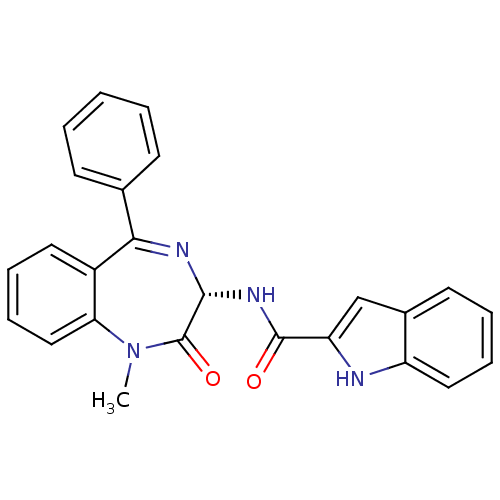
BDBM50005463 (R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl)-amide: 0.1C4H10O::(S)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl)-amide::(S)-N-(1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl)-1H-indole-2-carboxamide::(Z)-N-(1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl)-1H-indole-2-carboxamide::1H-Indole-2-carboxylic acid ((S)-1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl)-amide::1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl)-amide::1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl)-amide (L-364,718 ((S)-devazepide)::1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl)-amide (MK-329, L-364,718)::1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl)-amide(Devazepide or (R) L364718)::1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl)-amide(L-364718)::1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl)-amide(devazepide)::1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl)-amide: 0.1C4H10O. 0.15CH2Cl2::CCK antagonist synthetic 17::CCK antagonist synthetic 18::CHEMBL9506::DEVAZEPIDE::L-364,718::L-364718::MK-329
SMILES CN1c2ccccc2C(=N[C@H](NC(=O)c2cc3ccccc3[nH]2)C1=O)c1ccccc1
InChI Key InChIKey=NFHRQQKPEBFUJK-HSZRJFAPSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 67 hits for monomerid = 50005463
Found 67 hits for monomerid = 50005463
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
Rhone-Poulenc Rorer Central Research
Curated by PDSP Ki Database
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database