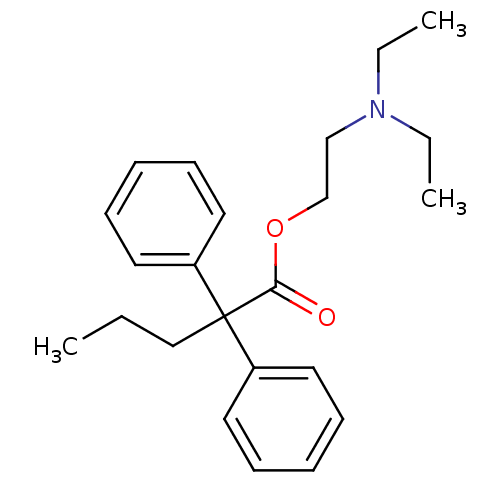
BDBM50017716 2,2-Diphenyl-pentanoic acid 2-diethylamino-ethyl ester::2-(diethylamino)ethyl 2,2-diphenylpentanoate::2-Benzhydryl-pentanoic acid 2-diethylamino-ethyl ester::CHEMBL282567::PROADIFEN HYDROCHLORIDE
SMILES CCCC(C(=O)OCCN(CC)CC)(c1ccccc1)c1ccccc1
InChI Key InChIKey=SNTQPLDRUZOSDP-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 14 hits for monomerid = 50017716
Found 14 hits for monomerid = 50017716
TargetSigma non-opioid intracellular receptor 1(Human)
Virginia Commonwealth University
Curated by ChEMBL
Virginia Commonwealth University
Curated by ChEMBL
Affinity DataKi: 17nMAssay Description:Binding affinity for Sigma receptor type 1,using [3H]-(+)-pentazocine as radioligandMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Human)
National Institutes of Health Chemical Genomics Center
Curated by ChEMBL
National Institutes of Health Chemical Genomics Center
Curated by ChEMBL
Affinity DataEC50: 2.51E+4nMAssay Description:Activation of human PXR expressed in human HepG2 (DPX-2) cells after 24 hrs by luciferase reporter gene based luminescent analysisMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Human)
National Institutes of Health Chemical Genomics Center
Curated by ChEMBL
National Institutes of Health Chemical Genomics Center
Curated by ChEMBL
Affinity DataEC50: 1.41E+4nMAssay Description:Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assayMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Human)
National Institutes of Health Chemical Genomics Center
Curated by ChEMBL
National Institutes of Health Chemical Genomics Center
Curated by ChEMBL
Affinity DataEC50: 1.26E+4nMAssay Description:Activation of human PXR expressed in human HepG2 (DPX-2) cells assessed as induction of CYP3A4 after 24 hrs by luminescent analysisMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Rat)
National Institutes of Health Chemical Genomics Center
Curated by ChEMBL
National Institutes of Health Chemical Genomics Center
Curated by ChEMBL
Affinity DataEC50: 3.55E+4nMAssay Description:Activation of rat PXR expressed in human HepG2 cells after 24 hrs by luciferase reporter gene based luminescent analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+4nMAssay Description:Inhibition of CYP450 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+5nMAssay Description:Inhibition of human aldehyde oxidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 4.20E+3nMAssay Description:Inhibition of binding of Batrachotoxinin [3H]BTX-B to high affinity sites on voltage dependent sodium channels in a vesicular preparation from guinea...More data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+4nMAssay Description:Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated ratsMore data for this Ligand-Target Pair
TargetSigma non-opioid intracellular receptor 1(Human)
Virginia Commonwealth University
Curated by ChEMBL
Virginia Commonwealth University
Curated by ChEMBL
Affinity DataIC50: 1.59E+3nMAssay Description:The compound was tested for affinity towards sigma-3 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 8.20E+3nMAssay Description:Inhibition of Aminopyrine N-demethylase in Phenobarbitone-treated ratsMore data for this Ligand-Target Pair
Affinity DataIC50: <1.32E+4nMAssay Description:Inhibition of human BSEP expressed in baculovirus transfected fall armyworm Sf21 cell membranes vesicles assessed as reduction in ATP-dependent [3H]-...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of rat aldehyde oxidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of rat aldehyde oxidaseMore data for this Ligand-Target Pair