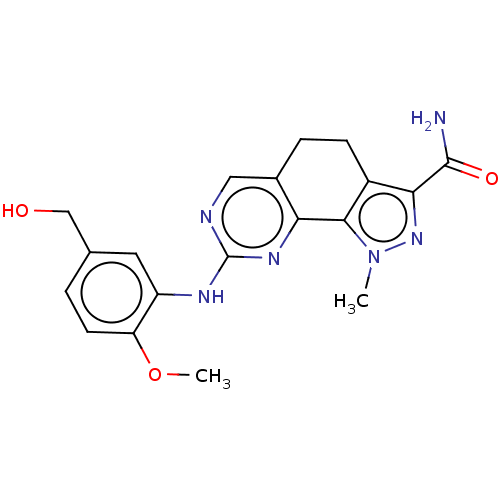
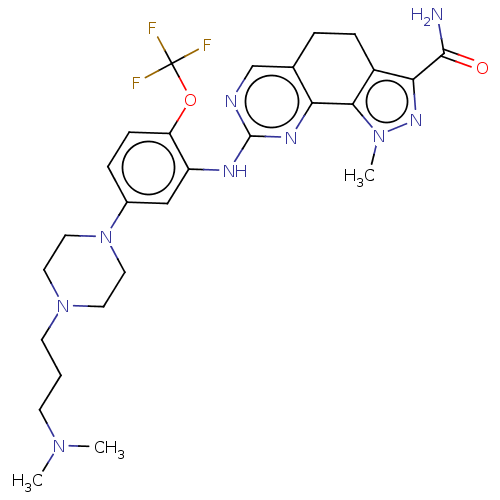
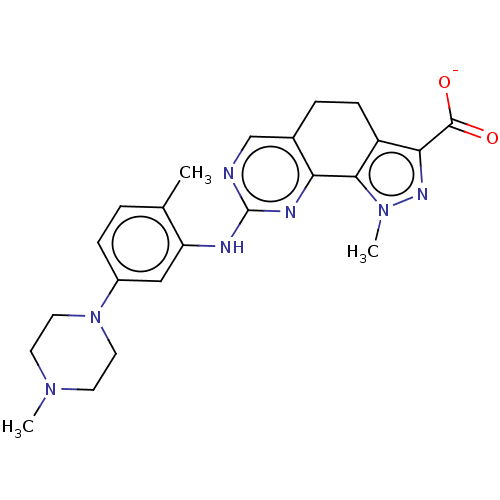
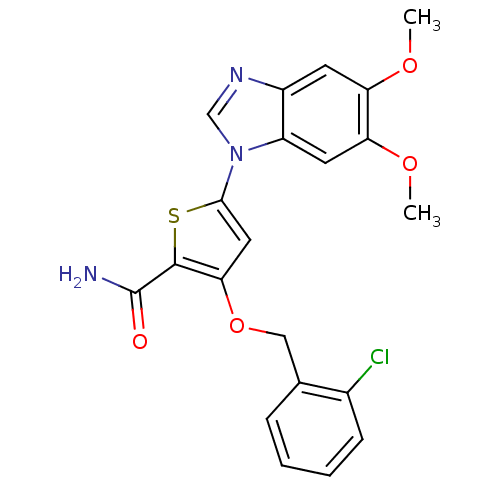
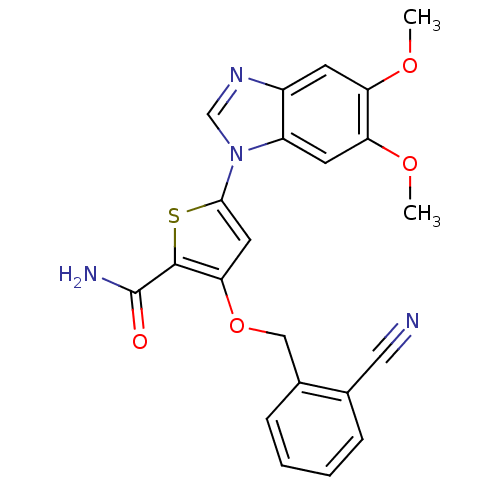
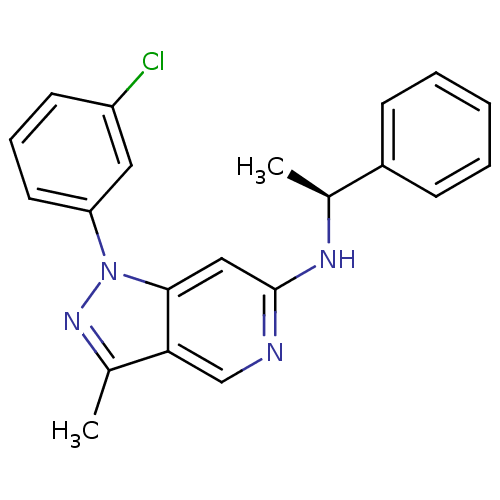
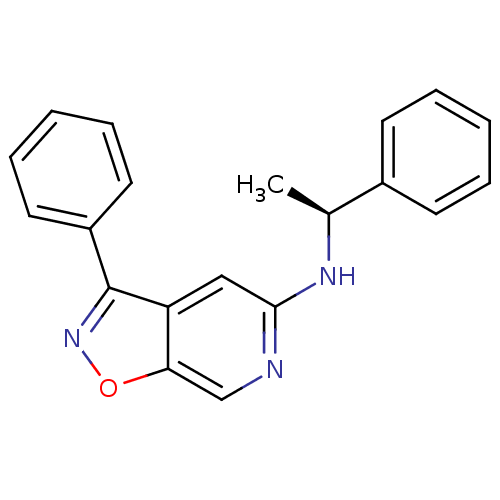
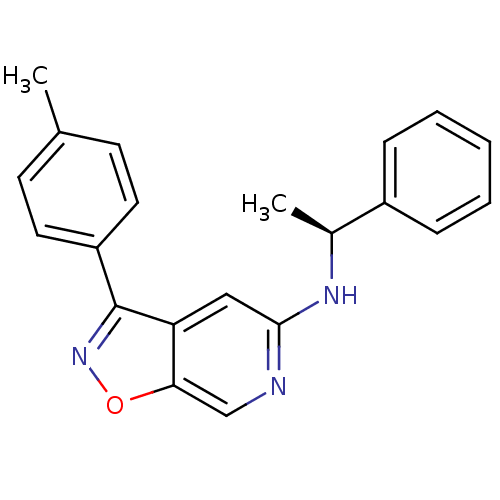
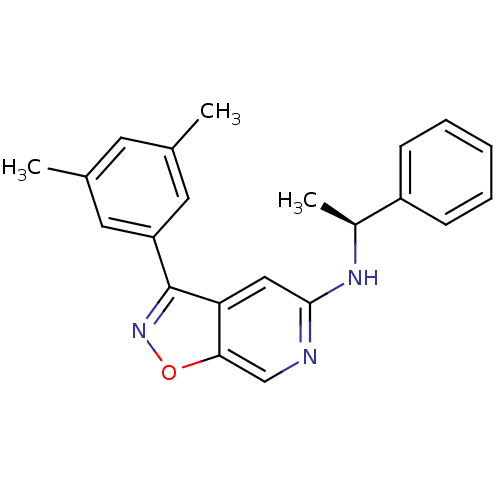
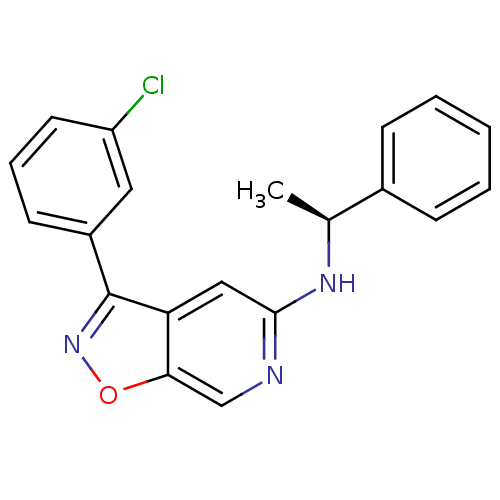
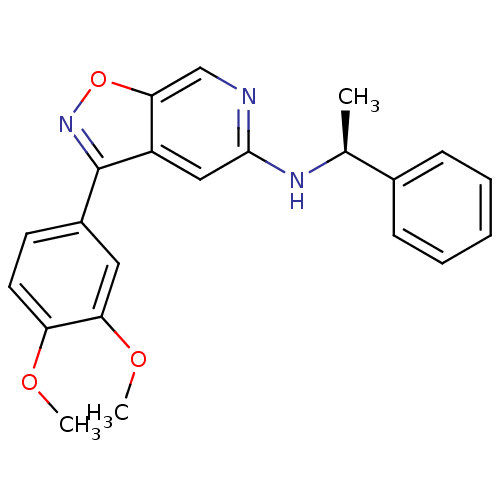
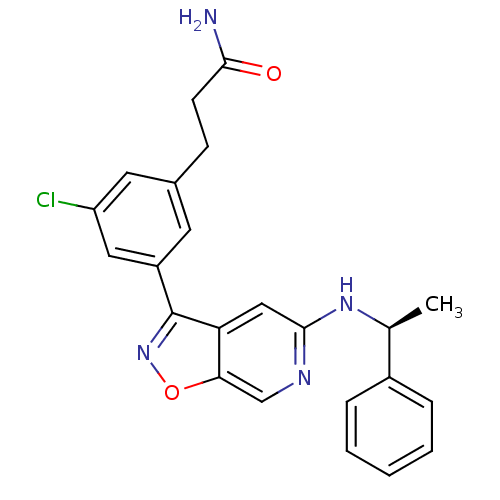
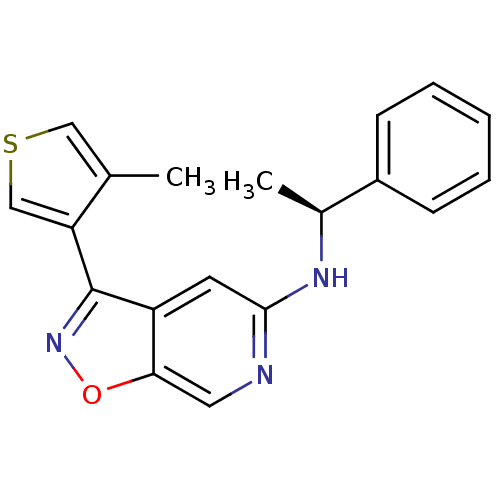
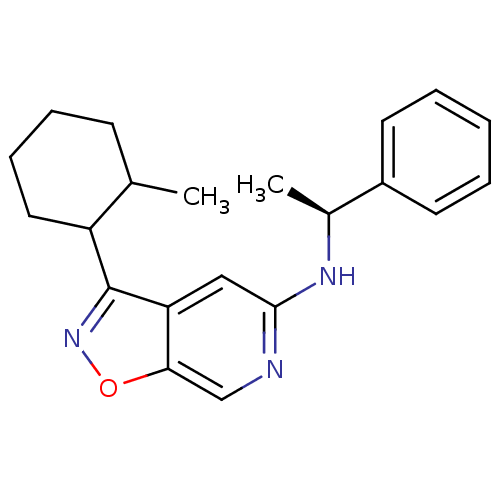
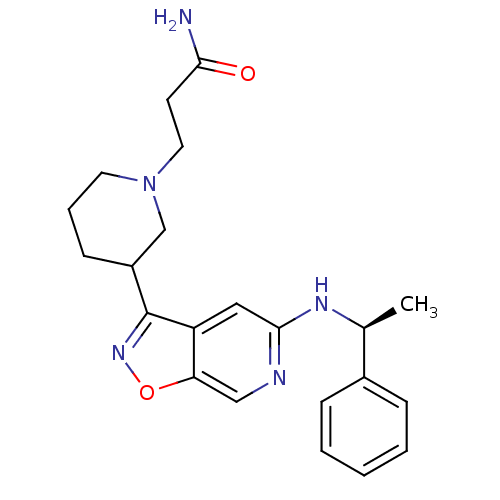
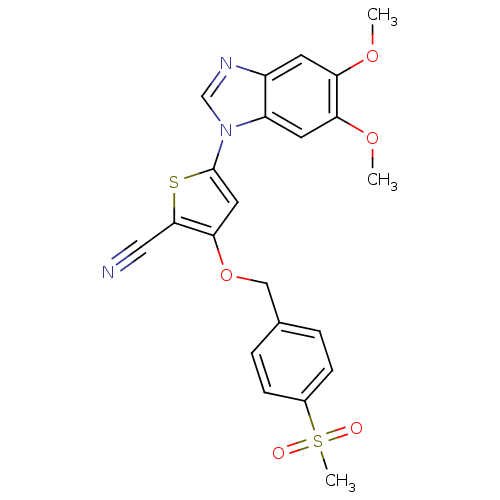
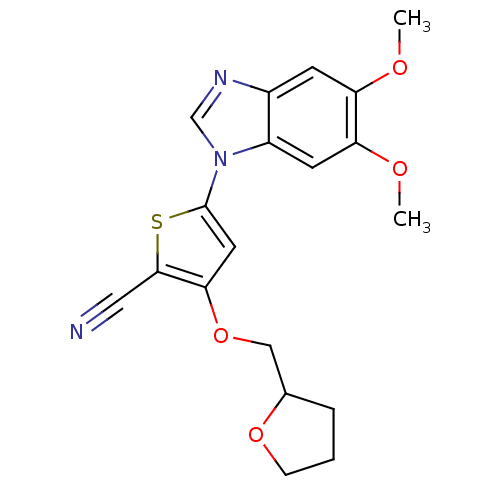
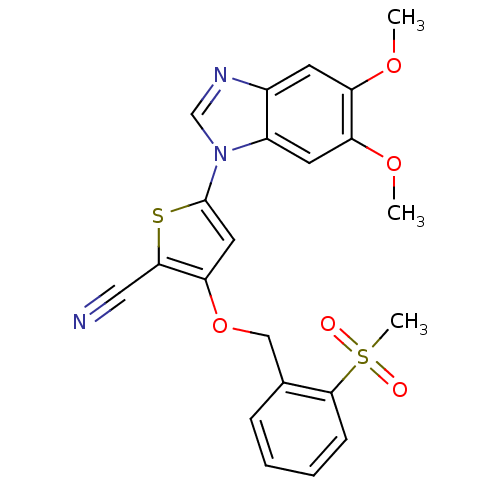
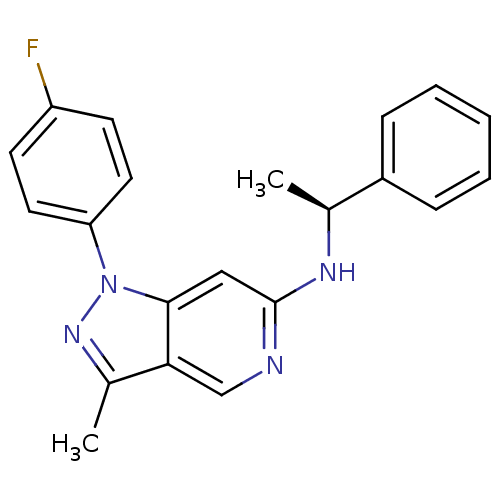
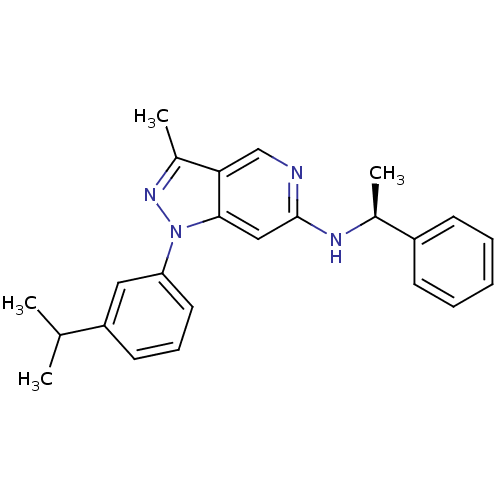
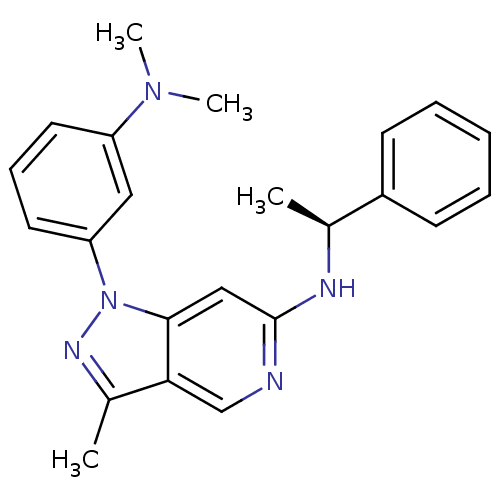
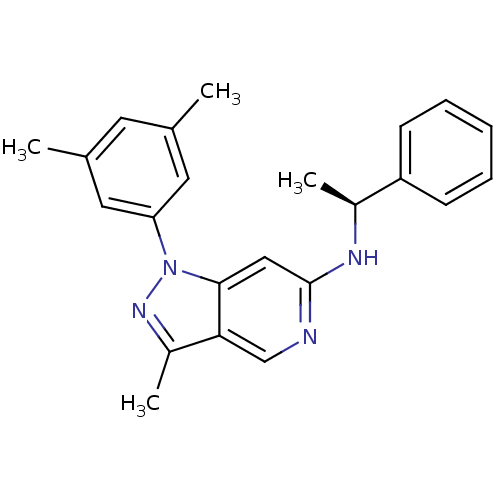
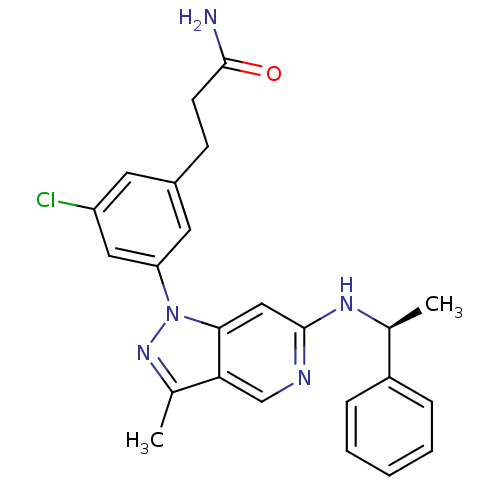
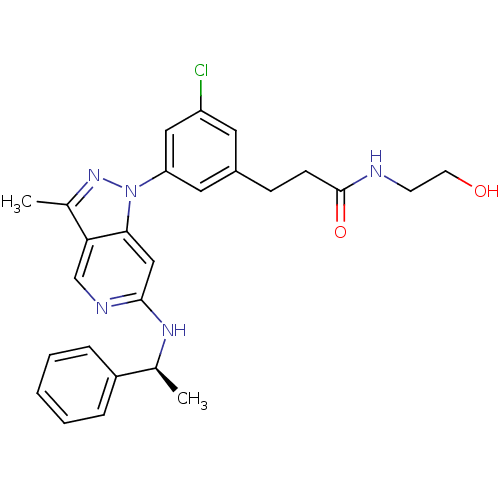
Affinity DataIC50: 10nMpH: 3.0Assay Description:The inhibitory activity of putative kinase inhibitors and the potency of selected compounds were determined using a trans-phosphorylation assay.Speci...More data for this Ligand-Target Pair
Affinity DataIC50: 26nMpH: 3.0Assay Description:The inhibitory activity of putative kinase inhibitors and the potency of selected compounds were determined using a trans-phosphorylation assay.Speci...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMpH: 3.0Assay Description:The inhibitory activity of putative kinase inhibitors and the potency of selected compounds were determined using a trans-phosphorylation assay.Speci...More data for this Ligand-Target Pair
Affinity DataIC50: 46nMpH: 3.0Assay Description:The inhibitory activity of putative kinase inhibitors and the potency of selected compounds were determined using a trans-phosphorylation assay.Speci...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMpH: 3.0Assay Description:The inhibitory activity of putative kinase inhibitors and the potency of selected compounds were determined using a trans-phosphorylation assay.Speci...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMpH: 7.2 T: 2°CAssay Description:The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMpH: 7.2 T: 2°CAssay Description:The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+3nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 121nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 1.62E+3nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 549nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 1.17E+3nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 330nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 625nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 4.93E+3nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 214nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 99nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 51nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 3.01E+3nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 779nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 5.69E+3nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 7.48E+3nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 126nMpH: 7.2 T: 2°CAssay Description:The compound inhibitory activity was determined by incubation with purified PLK1 and biotinylated peptide substrate in the presence ATP/[gamma-33P]AT...More data for this Ligand-Target Pair
Affinity DataIC50: 631nMpH: 7.2 T: 2°CAssay Description:The compound inhibitory activity was determined by incubation with purified PLK1 and biotinylated peptide substrate in the presence ATP/[gamma-33P]AT...More data for this Ligand-Target Pair
Affinity DataIC50: 251nMpH: 7.2 T: 2°CAssay Description:The compound inhibitory activity was determined by incubation with purified PLK1 and biotinylated peptide substrate in the presence ATP/[gamma-33P]AT...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMpH: 7.2 T: 2°CAssay Description:The compound inhibitory activity was determined by incubation with purified PLK1 and biotinylated peptide substrate in the presence ATP/[gamma-33P]AT...More data for this Ligand-Target Pair
Affinity DataIC50: 794nMpH: 7.2 T: 2°CAssay Description:The compound inhibitory activity was determined by incubation with purified PLK1 and biotinylated peptide substrate in the presence ATP/[gamma-33P]AT...More data for this Ligand-Target Pair
Affinity DataIC50: 126nMpH: 7.2 T: 2°CAssay Description:The compound inhibitory activity was determined by incubation with purified PLK1 and biotinylated peptide substrate in the presence ATP/[gamma-33P]AT...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+3nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 7.69E+3nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 474nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 4.53E+3nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 1.45E+4nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 5.93E+3nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 703nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 4.52E+3nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 464nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 3.24E+3nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 121nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 1.48E+3nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 412nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 977nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 225nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 149nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 641nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 274nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 21nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 32nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair
Affinity DataIC50: 48nMpH: 7.2 T: 2°CAssay Description:An IMAP fluorescence polarization-based assay format (Molecular Devices) was used to determine the ability of compounds to inhibit the phosphorylatio...More data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)