Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Hematopoietic prostaglandin D synthase
Ligand
BDBM21624
Substrate
BDBM21614
Meas. Tech.
In Vitro GST Activity Assay
pH
7.2±n/a
Temperature
295.15±n/a K
IC50
138±n/a nM
Citation
 Hohwy, M; Spadola, L; Lundquist, B; Hawtin, P; Dahmén, J; Groth-Clausen, I; Nilsson, E; Persdotter, S; von Wachenfeldt, K; Folmer, RH; Edman, K Novel prostaglandin d synthase inhibitors generated by fragment-based drug design. J Med Chem 51:2178-86 (2008) [PubMed] Article
Hohwy, M; Spadola, L; Lundquist, B; Hawtin, P; Dahmén, J; Groth-Clausen, I; Nilsson, E; Persdotter, S; von Wachenfeldt, K; Folmer, RH; Edman, K Novel prostaglandin d synthase inhibitors generated by fragment-based drug design. J Med Chem 51:2178-86 (2008) [PubMed] Article Target
Name:
Hematopoietic prostaglandin D synthase
Synonyms:
GSTS | Glutathione-dependent PGD synthetase | Glutathione-requiring prostaglandin D synthase | H-PGDS | HPGDS | HPGDS_HUMAN | Hematopoietic prostaglandin D synthase | Hematopoietic prostaglandin D synthase (H-PGDS) | Hematopoietic prostaglandin D synthase (HPGDS) | PGDS | PTGDS2 | Prostaglandin D | Prostaglandin D Synthase
Type:
Enzyme
Mol. Mass.:
23341.07
Organism:
Homo sapiens (Human)
Description:
The protein was expressed in E. coli strain BL21(DE3) with an N-terminal 6-His tag.
Residue:
199
Sequence:
MPNYKLTYFNMRGRAEIIRYIFAYLDIQYEDHRIEQADWPEIKSTLPFGKIPILEVDGLTLHQSLAIARYLTKNTDLAGNTEMEQCHVDAIVDTLDDFMSCFPWAEKKQDVKEQMFNELLTYNAPHLMQDLDTYLGGREWLIGNSVTWADFYWEICSTTLLVFKPDLLDNHPRLVTLRKKVQAIPAVANWIKRRPQTKL
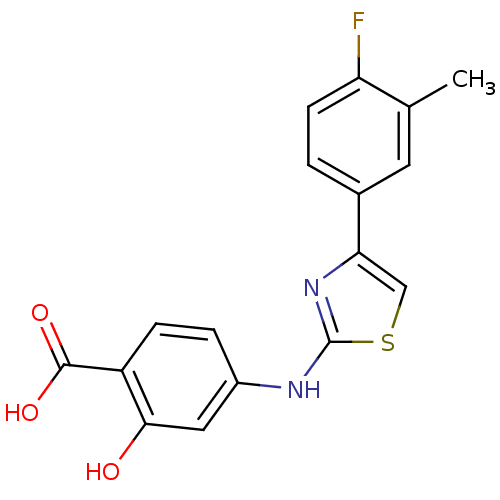
Inhibitor
Name:
BDBM21624
Synonyms:
4-{[4-(4-fluoro-3-methylphenyl)-1,3-thiazol-2-yl]amino}-2-hydroxybenzoic acid | Thiazolylamino, 12
Type:
Small organic molecule
Emp. Form.:
C17H13FN2O3S
Mol. Mass.:
344.36
SMILES:
Cc1cc(ccc1F)-c1csc(Nc2ccc(C(O)=O)c(O)c2)n1