Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Genome polyprotein
Ligand
BDBM27030
Substrate
NS3-NS4A Chromogenic Peptide Substrate
Meas. Tech.
Continuous Spectrophotometric Assay
Ki
3±n/a nM
Citation
 Venkatraman, S; Velazquez, F; Wu, W; Blackman, M; Chen, KX; Bogen, S; Nair, L; Tong, X; Chase, R; Hart, A; Agrawal, S; Pichardo, J; Prongay, A; Cheng, KC; Girijavallabhan, V; Piwinski, J; Shih, NY; Njoroge, FG Discovery and structure-activity relationship of P1-P3 ketoamide derived macrocyclic inhibitors of hepatitis C virus NS3 protease. J Med Chem 52:336-46 (2009) [PubMed] Article
Venkatraman, S; Velazquez, F; Wu, W; Blackman, M; Chen, KX; Bogen, S; Nair, L; Tong, X; Chase, R; Hart, A; Agrawal, S; Pichardo, J; Prongay, A; Cheng, KC; Girijavallabhan, V; Piwinski, J; Shih, NY; Njoroge, FG Discovery and structure-activity relationship of P1-P3 ketoamide derived macrocyclic inhibitors of hepatitis C virus NS3 protease. J Med Chem 52:336-46 (2009) [PubMed] Article More Info.:
Target
Name:
Genome polyprotein
Synonyms:
Dipeptidyl peptidase 4 (DPP4) | HCV NS3-NS4A Serine Proteinase | NS3 NS5B | NS3 Protease | NS3-4A Protease | NS3/4A Protein | NS3/4A protease | NS5B Polymerase | POLG_HCV77 | RNA polymerase (NS5B)
Type:
Protease Domain
Mol. Mass.:
327221.49
Organism:
Hepatitis C virus (HCV genotype 1a, isolate H)
Description:
P27958
Residue:
3011
Sequence:
MSTNPKPQRKTKRNTNRRPQDVKFPGGGQIVGGVYLLPRRGPRLGVRATRKTSERSQPRGRRQPIPKARRPEGRTWAQPGYPWPLYGNEGCGWAGWLLSPRGSRPSWGPTDPRRRSRNLGKVIDTLTCGFADLMGYIPLVGAPLGGAARALAHGVRVLEDGVNYATGNLPGCSFSIFLLALLSCLTVPASAYQVRNSSGLYHVTNDCPNSSVVYEAADAILHTPGCVPCVREGNASRCWVAVTPTVATRDGKLPTTQLRRHIDLLVGSATLCSALYVGDLCGSVFLVGQLFTFSPRHHWTTQDCNCSIYPGHITGHRMAWNMMMNWSPTAALVVAQLLRIPQAIMDMIAGAHWGVLAGIKYFSMVGNWAKVLVVLLLFAGVDAETHVTGGNAGRTTAGLVGLLTPGAKQNIQLINTNGSWHINSTALNCNESLNTGWLAGLFYQHKFNSSGCPERLASCRRLTDFAQGWGPISYANGSGLDERPYCWHYPPRPCGIVPAKSVCGPVYCFTPSPVVVGTTDRSGAPTYSWGANDTDVFVLNNTRPPLGNWFGCTWMNSTGFTKVCGAPPCVIGGVGNNTLLCPTDCFRKYPEATYSRCGSGPRITPRCMVDYPYRLWHYPCTINYTIFKVRMYVGGVEHRLEAACNWTRGERCDLEDRDRSELSPLLLSTTQWQVLPCSFTTLPALSTGLIHLHQNIVDVQYLYGVGSSIASWAIKWEYVVLLFLLLADARVCSCLWMMLLISQAEAALENLVILNAASLAGTHGLVSFLVFFCFAWYLKGRWVPGAVYALYGMWPLLLLLLALPQRAYALDTEVAASCGGVVLVGLMALTLSPYYKRYISWCMWWLQYFLTRVEAQLHVWVPPLNVRGGRDAVILLTCVVHPALVFDITKLLLAIFGPLWILQASLLKVPYFVRVQGLLRICALARKIAGGHYVQMAIIKLGALTGTCVYNHLAPLRDWAHNGLRDLAVAVEPVVFSRMETKLITWGADTAACGDIINGLPVSARRGQEILLGPADGMVSKGWRLLAPITAYAQQTRGLLGCIITSLTGRDKNQVEGEVQIVSTATQTFLATCINGVCWTVYHGAGTRTIASPKGPVIQTYTNVDQDLVGWPAPQGSRSLTPCTCGSSDLYLVTRHADVIPVRRRGDSRGSLLSPRPISYLKGSSGGPLLCPTGHAVGLFRAAVCTRGVAKAVDFIPVENLETTMRSPVFTDNSSPPAVPQSFQVAHLHAPTGSGKSTKVPAAYAAKGYKVLVLNPSVAATLGFGAYMSKAHGVDPNIRTGVRTITTGSPITYSTYGKFLADAGCSGGAYDIIICDECHSTDATSISGIGTVLDQAETAGARLVVLATATPPGSVTVSHPNIEEVALSTTGEIPFYGKAIPLEVIKGGRHLIFCHSKKKCDELAAKLVALGINAVAYYRGLDVSVIPTSGDVVVVSTDALMTGFTGDFDSVIDCNTCVTQTVDFSLDPTFTIETTTLPQDAVSRTQRRGRTGRGKPGIYRFVAPGERPSGMFDSSVLCECYDAGCAWYELTPAETTVRLRAYMNTPGLPVCQDHLGFWEGVFTGLTHIDAHFLSQTKQSGENFPYLVAYQATVCARAQAPPPSWDQMRKCLIRLKPTLHGPTPLLYRLGAVQNEVTLTHPITKYIMTCMSADLEVVTSTWVLVGGVLAALAAYCLSTGCVVIVGRIVLSGKPAIIPDREVLYQEFDEMEECSQHLPYIEQGMMLAEQFKQKALGLLQTASRHAEVITPAVQTNWQKLEVFWAKHMWNFISGIQYLAGLSTLPGNPAIASLMAFTAAVTSPLTTGQTLLFNILGGWVAAQLAAPGAATAFVGAGLAGAALDSVGLGKVLVDILAGYGAGVAGALVAFKIMSGEVPSTEDLVNLLPAILSPGALAVGVVFASILRRRVGPGEGAVQWMNRLIAFASRGNHVSPTHYVPESDAAARVTAILSSLTVTQLLRRLHQWISSECTTPCSGSWLRDIWDWICEVLSDFKTWLKAKLMPQLPGIPFVSCQRGYRGVWRGDGIMHTRCHCGAEITGHVKNGTMRIVGPRTCKNMWSGTFFINAYTTGPCTPLPAPNYKFALWRVSAEEYVEIRRVGDFHYVSGMTTDNLKCPCQIPSPEFFTELDGVRLHRFAPPCKPLLREEVSFRVGLHEYPVGSQLPCEPEPDVAVLTSMLTDPSHITAEAAGRRLARGSPPSMASSSASQLSAPSLKATCTANHDSPDAELIEANLLWRQEMGGNITRVESENKVVILDSFDPLVAEEDEREVSVPAEILRKSRRFAPALPVWARPDYNPLLVETWKKPDYEPPVVHGCPLPPPRSPPVPPPRKKRTVVLTESTLPTALAELATKSFGSSSTSGITGDNTTTSSEPAPSGCPPDSDVESYSSMPPLEGEPGDPDLSDGSWSTVSSGADTEDVVCCSMSYSWTGALVTPCAAEEQKLPINALSNSLLRHHNLVYSTTSRSACQRKKKVTFDRLQVLDSHYQDVLKEVKAAASKVKANLLSVEEACSLAPPHSAKSKFGYGAKDVRCHARKAVAHINSVWKDLLEDSVTPIDTTIMAKNEVFCVQPEKGGRKPARLIVFPDLGVRVCEKMALYDVVSKLPLAVMGSSYGFQYSPGQRVEFLVQAWKSKKTPMGLSYDTRCFDSTVTESDIRTEEAIYQCCDLDPQARVAIKSLTERLYVGGPLTNSRGENCGYRRCRASRVLTTSCGNTLTRYIKARAACRAAGLQDCTMLVCGDDLVVICESAGVQEDAASLRAFTEAMTRYSAPPGDPPQPEYDLELITSCSSNVSVAHDGAGKRVYYLTRDPTTPLARAAWETARHTPVNSWLGNIIMFAPTLWARMILMTHFFSVLIARDQLEQALNCEIYGACYSIEPLDLPPIIQRLHGLSAFSLHSYSPGEINRVAACLRKLGVPPLRAWRHRAWSVRARLLARGGKAAICGKYLFNWAVRTKLKLTPITAAGRLDLSGWFTAGYSGGDIYHSVSHARPRWFWFCLLLLAAGVGIYLLPNR
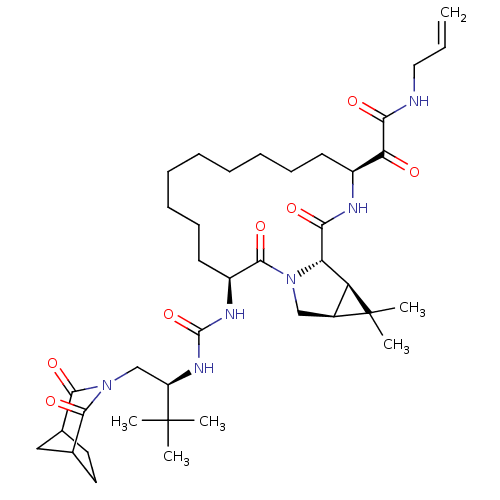
Inhibitor
Name:
BDBM27030
Synonyms:
2-[(3S,13S,16S,17R,19S)-3-({[(2S)-1-{2,4-dioxo-3-azabicyclo[3.2.1]octan-3-yl}-3,3-dimethylbutan-2-yl]carbamoyl}amino)-18,18-dimethyl-2,15-dioxo-1,14-diazatricyclo[14.4.0.0^{17,19}]icosan-13-yl]-2-oxo-N-(prop-2-en-1-yl)acetamide | ketoamide derived macrocyclic inhibitor, 54
Type:
Small organic molecule
Emp. Form.:
C39H60N6O7
Mol. Mass.:
724.9297
SMILES:
CC(C)(C)[C@@H](CN1C(=O)C2CCC(C2)C1=O)NC(=O)N[C@H]1CCCCCCCCC[C@H](NC(=O)[C@@H]2[C@@H]3[C@H](CN2C1=O)C3(C)C)C(=O)C(=O)NCC=C |r,TLB:15:14:10.11:13,THB:8:7:10.11:13,5:6:10.11:13|
Substrate
Name:
NS3-NS4A Chromogenic Peptide Substrate
Synonyms:
n/a
Type:
Peptide
Mol. Mass.:
1366.94
Organism:
n/a
Description:
The 4-phenylazophenol (PAP) chromophore was incorporated to improve assay sensitivity.
Residue:
13
Sequence:
ACDTEDVVPNVAH