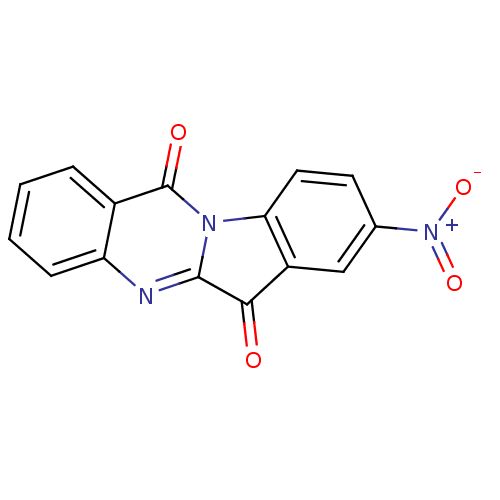
BDBM50442991 CHEMBL432537::GNF-Pf-3777::US10669273, Compound 5i
SMILES [O-][N+](=O)c1ccc-2c(c1)C(=O)c1nc3ccccc3c(=O)n-21
InChI Key InChIKey=UFMQJYHLIUACCG-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50442991
Found 3 hits for monomerid = 50442991
Affinity DataKi: 54nMAssay Description:Uncompetitive inhibition of human recombinant IDO1 using L-tryptophan as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0180nMAssay Description:Inhibition of human IDO1 expressed in HEK293 cells assessed as kynurenine release after 5 hrs by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 103nMAssay Description:Uncompetitive inhibition of human recombinant IDO1 using L-tryptophan as substrateMore data for this Ligand-Target Pair