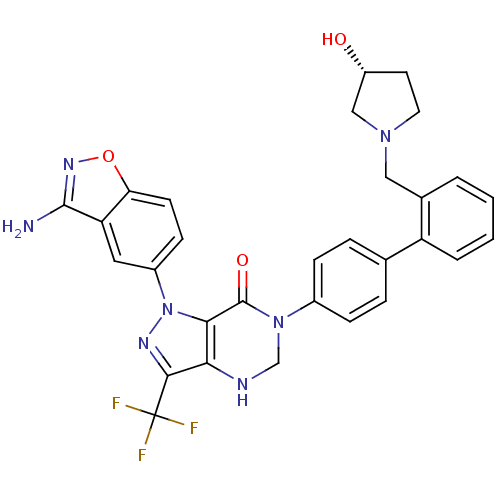
BDBM12868 1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-hydroxypyrrolidin-1-yl]methyl}phenyl)phenyl]-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[4,3-d]pyrimidin-7-one::dihydropyrazolo[4,3-d]pyrimidinone 24b
SMILES Nc1noc2ccc(cc12)-n1nc(c2NCN(C(=O)c12)c1ccc(cc1)-c1ccccc1CN1CC[C@@H](O)C1)C(F)(F)F
InChI Key InChIKey=KWXHTGARBNTADQ-OAQYLSRUSA-N
Data 3 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 12868
Found 3 hits for monomerid = 12868
TargetCoagulation factor X(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: 0.210nM ΔG°: -13.1kcal/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 130nM ΔG°: -9.29kcal/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
TargetCoagulation factor IX(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataKi: >4.10E+4nM ΔG°: >-5.98kcal/molepH: 7.4 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair