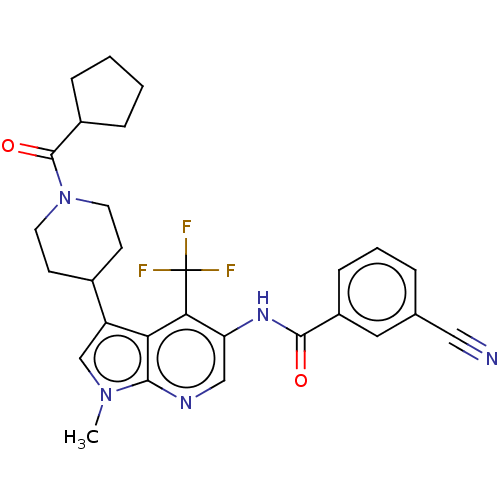
BDBM189897 US10227346, Example 23::US10426135, Example 23::US9670201, 23 3-cyano-N-(3-(1-(cyclopentanecarbonyl)piperidin-4-yl)-1-methyl-4-(trifluoromethyl)-1H-pyrrolo[2,3-b]pyridin-5-yl)benzamide::US9920054, Example 23
SMILES Cn1cc(C2CCN(CC2)C(=O)C2CCCC2)c2c(c(NC(=O)c3cccc(c3)C#N)cnc12)C(F)(F)F
InChI Key InChIKey=NZNIPTPCNOREKS-UHFFFAOYSA-N
Data 5 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 189897
Found 5 hits for monomerid = 189897
Affinity DataIC50: 4.70nMpH: 7.5 T: 2°CAssay Description:Specifically, in one embodiment the aforementioned assay was performed as outlined below. The assay was carried out in black polystyrene, 384-well pl...More data for this Ligand-Target Pair
Affinity DataIC50: 4.70nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 4.70nMAssay Description:Inhibition of His-tagged human RORgammat LBD in presence of biotin-coactivator peptide SRC1 binding incubated for 1 hr by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair