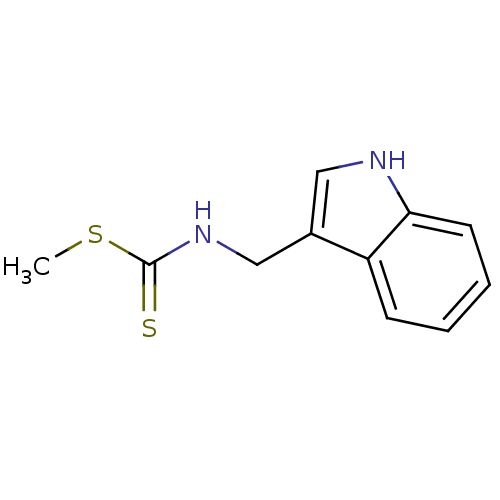
BDBM24813 Brassinin, 1::N-(1H-indol-3-ylmethyl)(methylsulfanyl)carbothioamide::methyl N-(1H-indol-3-ylmethyl)carbamodithioate
SMILES CSC(=S)NCc1c[nH]c2ccccc12
InChI Key InChIKey=QYKQWFZDEDFELK-UHFFFAOYSA-N
Data 4 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 24813
Found 4 hits for monomerid = 24813
Affinity DataKi: 9.77E+4nMAssay Description:Binding affinity to IDO1 (unknown origin) assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKi: 9.77E+4nM ΔG°: -5.69kcal/molepH: 6.5 T: 2°CAssay Description:The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr...More data for this Ligand-Target Pair
Affinity DataKi: 9.80E+4nMAssay Description:Inhibition of human recombinant IDO assessed as inhibition of indoleamine 2,3-dioxygenase to kynurenine conversion after 60 mins by HPLC analysisMore data for this Ligand-Target Pair