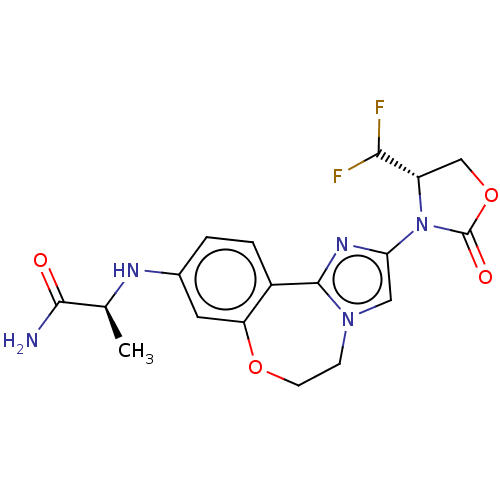
BDBM295665 (S)-2-((2-((S)-4-(difluoromethyl)- 2-oxooxazolidin-3-yl)-5,6- dihydrobenzo[f]imidazo[1,2- d][1,4]oxazepin-9- yl)amino)propanamide::US10112932, Compound 101::US10851091, Compound 101
SMILES C[C@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)F)C(N)=O
InChI Key InChIKey=SGEUNORSOZVTOL-CABZTGNLSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 19 hits for monomerid = 295665
Found 19 hits for monomerid = 295665
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0340nMAssay Description:PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0340nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0340nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 12.2nMAssay Description:PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 12.2nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 18nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 18.2nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 18.2nMAssay Description:PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 99.7nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
Affinity DataKi: 99.7nMAssay Description:PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 100nMAssay Description:Inhibition of PI3Kbeta (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP2C8 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataEC50: 19nMAssay Description:Inhibition of PI3Kalpha H1047R mutant in human HCC1954 cells assessed as reduction in PRAS40 phosphorylation after 24 hrs by electrochemiluminescent ...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)