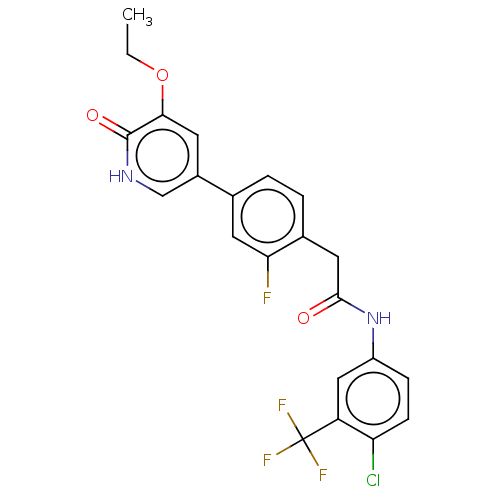
BDBM347328 N-(4-Chloro-3-(trifluoromethyl)phenyl)-2-(4-(5-ethoxy-6-oxo-1,6-dihydropyridin-3-yl)-2-fluorophenyl)acetamide::US9789100, Example 16
SMILES CCOc1cc(c[nH]c1=O)-c1ccc(CC(=O)Nc2ccc(Cl)c(c2)C(F)(F)F)c(F)c1
InChI Key InChIKey=ZWBVCWWHGLDFQI-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 347328
Found 3 hits for monomerid = 347328
TargetProto-oncogene tyrosine-protein kinase receptor Ret [658-1114](Homo sapiens (Human))
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataIC50: <100nMAssay Description:Human RET kinase cytoplasmic domain (amino acids 658-1114 of accession number NP_000314.1) was expressed as an N-terminal GST-fusion protein using a ...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret [658-1114](Homo sapiens (Human))
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataIC50: <100nMAssay Description:Human RET kinase cytoplasmic domain (amino acids 658-1114 of accession number NP_000314.1) was expressed as an N-terminal GST-fusion protein using a ...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret [658-1114](Homo sapiens (Human))
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataIC50: <100nMAssay Description:Human RET kinase cytoplasmic domain (amino acids 658-1114 of accession number NP_000314.1) was expressed as an N-terminal GST-fusion protein using a ...More data for this Ligand-Target Pair