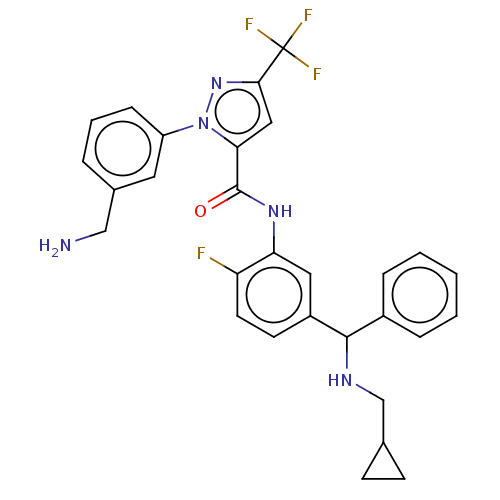
BDBM416773 1-(3-(aminomethyl)phenyl)-N-(5-(((cyclopropylmethyl)amino)(phenyl)methyl)-2-fluorophenyl)-3-(trifluoromethyl)-1H-pyrazole-5-carboxamide::US10329260, Compound 38d::US10633345, Compound 38d::US10689346, Compound 42a::US11203574, Compound 42a::US11230530, Compound 42a::US11685721, Compound 38d::US11708332, Compound 38d
SMILES NCc1cccc(c1)-n1nc(cc1C(=O)Nc1cc(ccc1F)C(NCC1CC1)c1ccccc1)C(F)(F)F
InChI Key InChIKey=MCLZOCFEIKBMBA-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 31 hits for monomerid = 416773
Found 31 hits for monomerid = 416773
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:Plasma kallikrein activity assay. The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:Plasma kallikrein activity assay. The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:Plasma kallikrein activity assay. The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates. In these experiments, 2...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates. In these experiments, 2...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates. In these experiments, 2...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:Plasma kallikrein activity assay. The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:Plasma kallikrein activity assay. The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic s...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:Plasma kallikrein activity assay. The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic s...More data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of purified human plasma kallikrein using H-D-Pro-Phe-Arg-pNA.2HCl as substrate measured after 3 mins by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of purified human plasma kallikrein using H-D-Pro-Phe-Arg-pNA.2HCl as substrate measured after 3 mins by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of purified human plasma kallikrein using H-D-Pro-Phe-Arg-pNA.2HCl as substrate measured after 3 mins by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of human plasma kallikrein using H-D-Pro-Phe-Arg-p-nitroaniline as substrate assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of endogenous human plasma kallikrein using Z-Phe-Arg-AMC.HCl as substrate measured after 5 mins by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of endogenous human plasma kallikrein using Z-Phe-Arg-AMC.HCl as substrate measured after 5 mins by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of endogenous human plasma kallikrein using Z-Phe-Arg-AMC.HCl as substrate measured after 5 mins by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Inhibition of purified human plasma kallikrein using H-D-Pro-Phe-Arg-pNA.2HCl as substrate measured after 3 mins by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 8.51nMAssay Description:Inhibition of endogenous human plasma kallikrein using Z-Phe-Arg-AMC.HCl as substrate measured after 5 mins by microplate reader analysisMore data for this Ligand-Target Pair