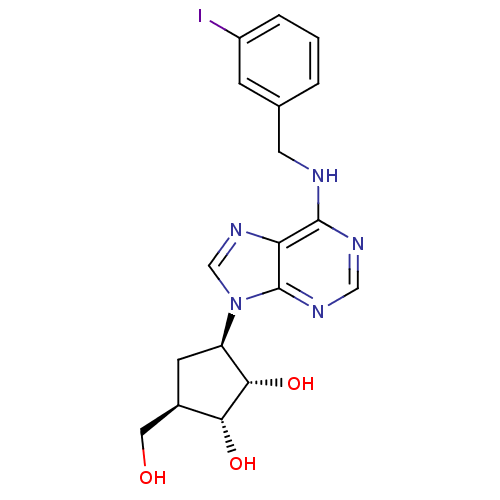
BDBM50088420 3-Hydroxymethyl-5-[6-(3-iodo-benzylamino)-purin-9-yl]-cyclopentane-1,2-diol::CHEMBL71385
SMILES OC[C@H]1C[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)ncnc12
InChI Key InChIKey=JIBVHQBGLJITFH-CUBALJKWSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50088420
Found 4 hits for monomerid = 50088420
Affinity DataKi: 1.96E+3nMAssay Description:Displacement of [125I]- AB-MECA from human adenosine A3 receptor expressed in HEK cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2.59E+4nMAssay Description:Displacement of [3H]R-PIA from rat brain membrane Adenosine A1 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Stimulation of [35S]GTP-gamma-S, against human adenosine A3 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Stimulation of [35S]GTP-gamma-S, binding to human adenosine A1 receptorMore data for this Ligand-Target Pair