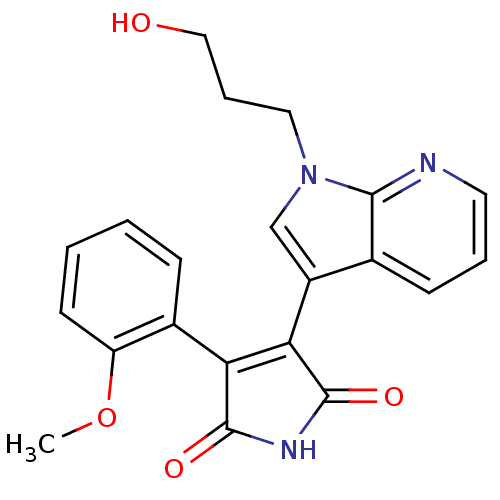
BDBM50147472 3-[1-(3-Hydroxy-propyl)-1H-pyrrolo[2,3-b]pyridin-3-yl]-4-(2-methoxy-phenyl)-pyrrole-2,5-dione::CHEMBL111298
SMILES COc1ccccc1C1=C(C(=O)NC1=O)c1cn(CCCO)c2ncccc12
InChI Key InChIKey=PDZRGSNSFAAGRB-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50147472
Found 3 hits for monomerid = 50147472
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrateMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of human Protein kinase C alphaMore data for this Ligand-Target Pair
TargetProtein kinase C beta type(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.44E+3nMAssay Description:Inhibitory concentration against human protein kinase C-betaII using histone as substrateMore data for this Ligand-Target Pair