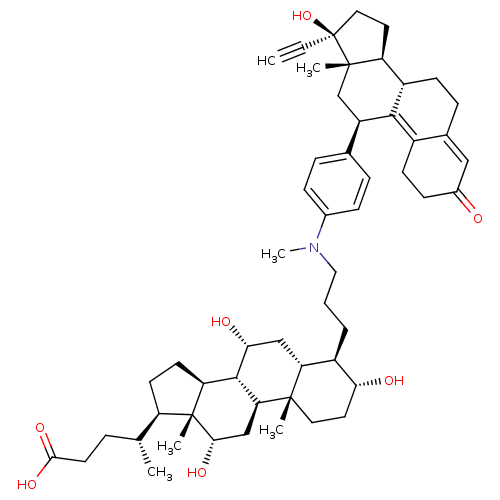
BDBM50151072 (4R)-4-[(1S,2S,5R,6R,7R,9R,10R,11S,14R,15R,16S)-6-[3-({4-[(10S,11S,14R,15S,17R)-14-ethynyl-14-hydroxy-15-methyl-5-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-1,6-dien-17-yl]phenyl}(methyl)amino)propyl]-5,9,16-trihydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-14-yl]pentanoic acid::CHEMBL440251
SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4[C@@H](CCCN(C)c5ccc(cc5)[C@H]5C[C@@]6(C)[C@@H](CC[C@@]6(O)C#C)[C@@H]6CCC7=CC(=O)CCC7=C56)[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C
InChI Key InChIKey=QIWQUMBYZAMEPN-NFCXHWEESA-N
Data 8 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50151072
Found 8 hits for monomerid = 50151072
Affinity DataKi: 0.800nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2.10nMAssay Description:Inhibition of human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataKi: 46nMAssay Description:Inhibition of human Estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataKi: 48nMAssay Description:Inhibition of human Estrogen receptor betaMore data for this Ligand-Target Pair
Affinity DataKi: 52nMAssay Description:Inhibition of glucocorticoid receptor dependent alkaline phosphatase activityMore data for this Ligand-Target Pair
Affinity DataKi: 53nMAssay Description:Inhibition of human androgen receptorMore data for this Ligand-Target Pair
Affinity DataKi: 340nMAssay Description:Inhibition of glucocorticoid receptor mediated tyrosine amino transferase activityMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of human Mineralocorticoid receptorMore data for this Ligand-Target Pair