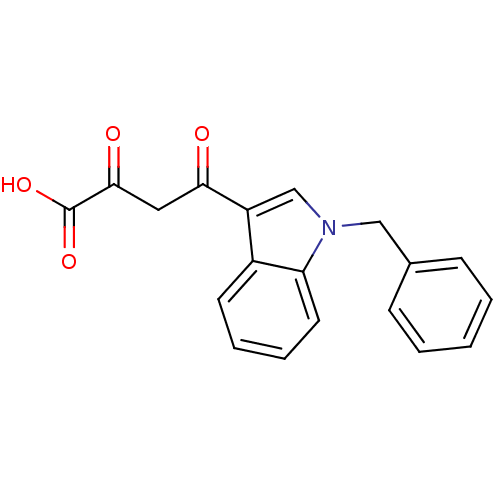
BDBM50154408 (Z)-4-(1-Benzyl-1H-indol-3-yl)-2-hydroxy-4-oxo-but-2-enoic acid::CHEMBL1673089::CHEMBL186820
SMILES OC(=O)C(=O)CC(=O)c1cn(Cc2ccccc2)c2ccccc12
InChI Key InChIKey=KXWMICWZAOIFIN-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50154408
Found 9 hits for monomerid = 50154408
Affinity DataKi: 1.93E+3nMAssay Description:Inhibition of human CA2 using CO2 as substrate incubated for 6 hrs prior to substrate addition measured for 10 to 100 secs by stopped flow techniqueMore data for this Ligand-Target Pair
Affinity DataKi: 2.78E+3nMAssay Description:Inhibition of human CA1 using CO2 as substrate incubated for 6 hrs prior to substrate addition measured for 10 to 100 secs by stopped flow techniqueMore data for this Ligand-Target Pair
Affinity DataKi: 7.64E+3nMAssay Description:Inhibition of human CA12 using CO2 as substrate incubated for 6 hrs prior to substrate addition measured for 10 to 100 secs by stopped flow techniqueMore data for this Ligand-Target Pair
Affinity DataKi: 8.28E+3nMAssay Description:Inhibition of human CA9 using CO2 as substrate incubated for 6 hrs prior to substrate addition measured for 10 to 100 secs by stopped flow techniqueMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:In vitro concentration required to inhibit the overall HIV-1 integrase strand transferMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:In vitro concentration required to inhibit the HIV-1 integrase strand transferMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated 3' processing reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated strand transfer reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 8.28E+3nMAssay Description:In vitro concentration required to inhibit the HIV-1 integrase 3' strand transferMore data for this Ligand-Target Pair