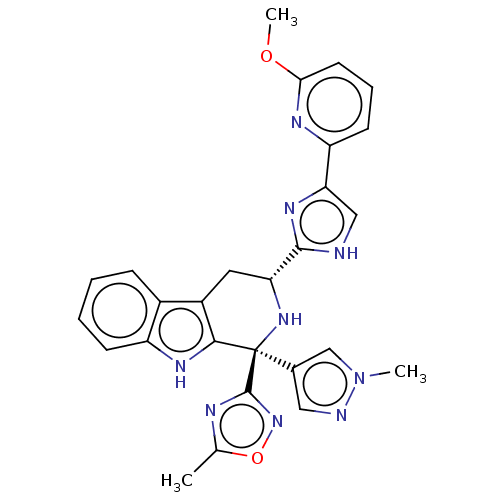
BDBM50154423 CHEMBL3774684
SMILES COc1cccc(n1)-c1c[nH]c(n1)[C@H]1Cc2c([nH]c3ccccc23)[C@](N1)(c1cnn(C)c1)c1noc(C)n1
InChI Key InChIKey=MBFARSZGJXRMQQ-JIPXPUAJSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50154423
Found 3 hits for monomerid = 50154423
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3.62E+3nMAssay Description:Binding affinity to human ERGMore data for this Ligand-Target Pair
TargetSomatostatin receptor type 3(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:Binding affinity to human somatostatin receptor type 3More data for this Ligand-Target Pair
TargetSomatostatin receptor type 3(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataEC50: 4.80nMAssay Description:Antagonist activity at human somatostatin receptor type 3 assessed as inhibition of cAMP levelsMore data for this Ligand-Target Pair