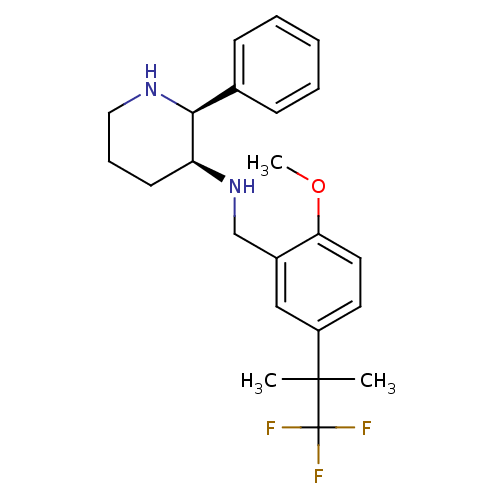
BDBM50224198 (2S,3S)-N-(2-methoxy-5-(1,1,1-trifluoro-2-methylpropan-2-yl)benzyl)-2-phenylpiperidin-3-amine::(S,3S)-3-[5-(1,1-Dimethyl-2,2,2-trifluoroethyl)-2-methoxybenzylamino]-2-phenylpiperidine::CHEMBL392368
SMILES COc1ccc(cc1CN[C@H]1CCCN[C@H]1c1ccccc1)C(C)(C)C(F)(F)F
InChI Key InChIKey=WUTRELIDULYHSO-FPOVZHCZSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50224198
Found 4 hits for monomerid = 50224198
Affinity DataKi: 840nMAssay Description:Binding affinity to human CYP2D6 using bufuralol as substrateMore data for this Ligand-Target Pair
TargetSubstance-P receptor(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Antagonist activity at neurokinin K1 receptorMore data for this Ligand-Target Pair
TargetSubstance-P receptor(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Displacement of [3H]spiperone from NK1 receptor in human IM9 cellsMore data for this Ligand-Target Pair
TargetSubstance-P receptor(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Antagonist activity at neurokinin 1 receptorMore data for this Ligand-Target Pair