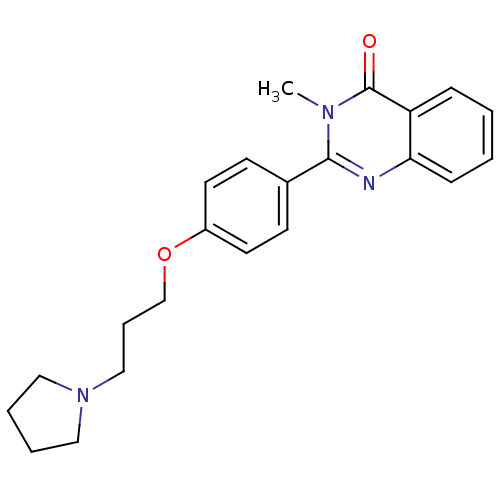
BDBM50246289 3-methyl-2-(4-(3-(pyrrolidin-1-yl)propoxy)phenyl)quinazolin-4(3H)-one::CHEMBL488248::CHEMBL557214
SMILES Cn1c(nc2ccccc2c1=O)-c1ccc(OCCCN2CCCC2)cc1
InChI Key InChIKey=PRPSMPHMELIHFQ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 14 hits for monomerid = 50246289
Found 14 hits for monomerid = 50246289
Affinity DataKi: 0.219nMAssay Description:Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.40nMAssay Description:Displacement of 3-([1,1,1-3H]methyl)-2-(4-{[3-(1-pyrrolidinyl)propyl]oxy}phenyl)-4(3H)-quinazolinone from mouse histamine H3 receptor expressed in HE...More data for this Ligand-Target Pair
Affinity DataKi: 4.20nMAssay Description:Binding affinity to human histamine H3 receptor by competitive binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Binding affinity to rat histamine H3 receptor by competitive binding assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Bioprojet-Biotech
Curated by ChEMBL
Bioprojet-Biotech
Curated by ChEMBL
Affinity DataIC50: 2.90E+3nMAssay Description:Displacement of [3H]dofetilide from human recombinant ERG by Competitive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of histamine H2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of histamine H4 receptorMore data for this Ligand-Target Pair
Affinity DataKd: 2.60nMAssay Description:Binding affinity to mouse histamine H3 receptor overexpressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKd: 3.10nMAssay Description:Binding affinity to histamine H3 receptor in wild type mouse brain membraneMore data for this Ligand-Target Pair
Affinity DataEC50: 9.60nMAssay Description:Inverse agonist activity at mouse histamine H3 receptor overexpressed in HEK293 cells by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMAssay Description:Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Bioprojet-Biotech
Curated by ChEMBL
Bioprojet-Biotech
Curated by ChEMBL
Affinity DataIC50: 2.90E+3nMAssay Description:Displacement of [35S]MK499 from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.680nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of histamine H1 receptorMore data for this Ligand-Target Pair