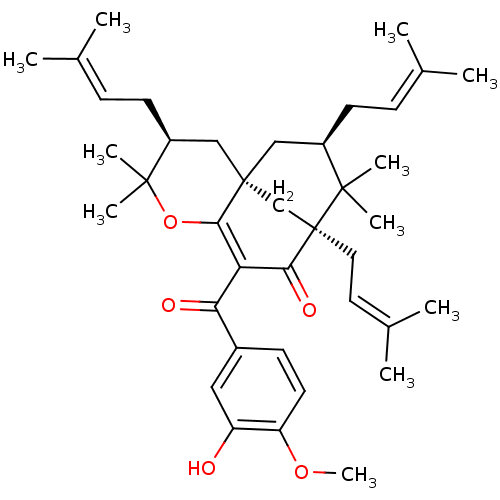
BDBM50265449 (1R,3S,9S,11R)-7-(3-Hydroxy-4-methoxy-benzoyl)-4,4,10,10-tetramethyl-3,9,11-tris-(3-methyl-but-2-enyl)-5-oxa-tricyclo[7.3.1.0*1,6*]tridec-6-en-8-one::CHEMBL500215
SMILES [#6]-[#8]-c1ccc(cc1-[#8])-[#6](=O)-[#6]-1=[#6]2-[#8]C([#6])([#6])[#6@@H](-[#6]\[#6]=[#6](\[#6])-[#6])-[#6][C@@]22[#6]-[#6@@H](-[#6]\[#6]=[#6](\[#6])-[#6])C([#6])([#6])[C@]([#6]\[#6]=[#6](\[#6])-[#6])([#6]2)[#6]-1=O
InChI Key InChIKey=SSFAIITVXFSDBR-HSFDOICUSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50265449
Found 3 hits for monomerid = 50265449
TargetHistone acetyltransferase p300(Homo sapiens (Human))
Jawaharlal Nehru Centre For Advanced Scientific Research
Curated by ChEMBL
Jawaharlal Nehru Centre For Advanced Scientific Research
Curated by ChEMBL
Affinity DataKi: 5.10E+3nMAssay Description:Inhibition of HAT p300 catalytic domain by equilibrium dialysisMore data for this Ligand-Target Pair
TargetHistone acetyltransferase p300(Homo sapiens (Human))
Jawaharlal Nehru Centre For Advanced Scientific Research
Curated by ChEMBL
Jawaharlal Nehru Centre For Advanced Scientific Research
Curated by ChEMBL
Affinity DataKd: 1.40E+3nMAssay Description:Binding affinity to HAT p300 catalytic domain by fluorometric titrationMore data for this Ligand-Target Pair
TargetHistone acetyltransferase p300(Homo sapiens (Human))
Jawaharlal Nehru Centre For Advanced Scientific Research
Curated by ChEMBL
Jawaharlal Nehru Centre For Advanced Scientific Research
Curated by ChEMBL
Affinity DataKd: 9.10E+3nMAssay Description:Binding affinity to HAT p300 catalytic domain by isothermal titration calorimetryMore data for this Ligand-Target Pair