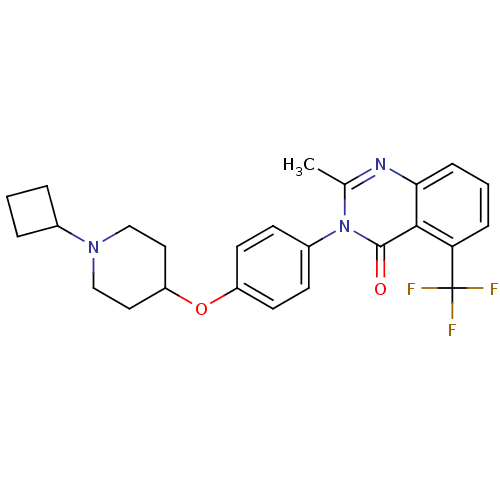
BDBM50274235 3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2-methyl-5-(trifluoromethyl)-4(3H)-quinazolinone::CHEMBL485543
SMILES Cc1nc2cccc(c2c(=O)n1-c1ccc(OC2CCN(CC2)C2CCC2)cc1)C(F)(F)F
InChI Key InChIKey=ZWUFGBNOUKQGBR-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50274235
Found 8 hits for monomerid = 50274235
Affinity DataKi: 2.30nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3.60nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3.90nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from mouse recombinant histamine H3 receptorMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Antagonist activity at human ERG in HEK293 cells assessed as inhibition of [35S]N-[(4R)-1'-[(2R)-6-cyano-1,2,3,4-tetrahydro-2-naphthalenyl]-3,4-dihyd...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human histamine H4 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human histamine H2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.330nMAssay Description:Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT...More data for this Ligand-Target Pair