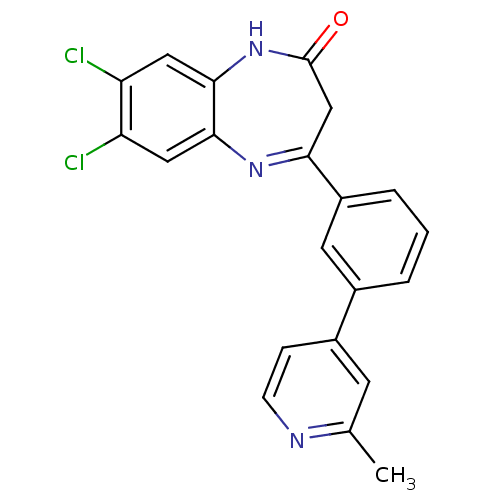
BDBM50332966 7,8-dichloro-4-(3-(2-methylpyridin-4-yl)phenyl)-1H-benzo[b][1,4]diazepin-2(3H)-one::CHEMBL1629864
SMILES Cc1cc(ccn1)-c1cccc(c1)C1=Nc2cc(Cl)c(Cl)cc2NC(=O)C1
InChI Key InChIKey=CMLVJJVYIVBZNH-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50332966
Found 4 hits for monomerid = 50332966
Affinity DataIC50: 2nMAssay Description:Antagonist activity at recombinant rat mGluR2 expressed in forskolin-stimulated CHO cells assessed as inhibition of (1S,3R)-ACPD induced cAMP product...More data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 3(Rattus norvegicus (Rat))
F. Hoffmann-La Roche
Curated by ChEMBL
F. Hoffmann-La Roche
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Antagonist activity at rat mGluR3 receptor expressed in CHO cells assessed as inhibition of GIRK currentMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Antagonist activity at human mGluR2 receptor expressed in CHO cells assessed as inhibition of GIRK currentMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Partial displacement of [3H]LY354740 from recombinant rat mGluR2More data for this Ligand-Target Pair