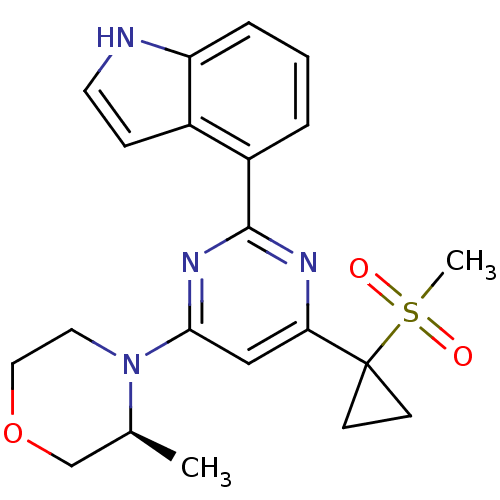
BDBM50427329 CHEMBL2325696
SMILES C[C@H]1COCCN1c1cc(nc(n1)-c1cccc2[nH]ccc12)C1(CC1)S(C)(=O)=O
InChI Key InChIKey=SCGCBAAYLFTIJU-AWEZNQCLSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50427329
Found 2 hits for monomerid = 50427329
Affinity DataIC50: 3.70E+3nMAssay Description:Inhibition of ATR-mediated CHK1 phosphorylation at serine 345 in human HT29 cells after 1 hr in presence of 4-nitroquinoline 1-oxideMore data for this Ligand-Target Pair
Affinity DataIC50: 34nMAssay Description:Inhibition of ATR in human HeLa cell nuclear extracts using glutathione S-transferase-p53N66 and ATP as substrate incubated for 10 mins prior to ATP ...More data for this Ligand-Target Pair