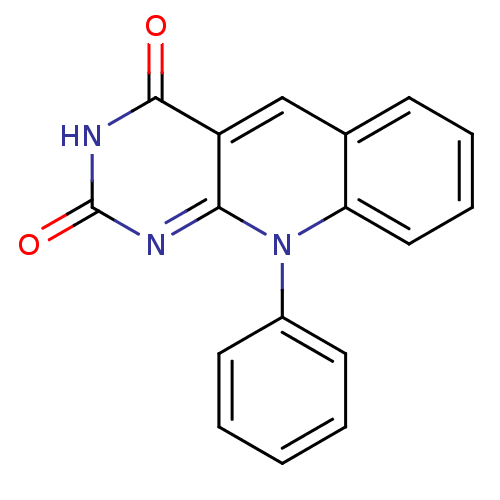
BDBM50438882 10-Phenylpyrimido[4,5-b]quinoline-2,4(3H,10H)-dione (5)::CHEMBL471342
SMILES O=c1nc2n(-c3ccccc3)c3ccccc3cc2c(=O)[nH]1
InChI Key InChIKey=BBWFTVFLVHVBCD-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50438882
Found 9 hits for monomerid = 50438882
TargetDNA-(apurinic or apyrimidinic site) endonuclease(Homo sapiens (Human))
University Of Manchester
Curated by ChEMBL
University Of Manchester
Curated by ChEMBL
Affinity DataIC50: >2.00E+5nMAssay Description:Inhibition of APE-1 (unknown origin) using double-stranded AP-site containing DNA as substrate after 30 mins by fluorescence assayMore data for this Ligand-Target Pair
TargetTyrosyl-DNA phosphodiesterase 2(Homo sapiens (Human))
University Of Manchester
Curated by ChEMBL
University Of Manchester
Curated by ChEMBL
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of TDP2 (unknown origin) using 5'-Y-TCCGTTGAAGCCTGCTTT-3' as substrate after 60 minsMore data for this Ligand-Target Pair
TargetTyrosyl-DNA phosphodiesterase 2(Homo sapiens (Human))
University Of Manchester
Curated by ChEMBL
University Of Manchester
Curated by ChEMBL
Affinity DataEC50: >3.00E+4nMAssay Description:Inhibition of TDP2 (unknown origin) using 4-nitrophenyl phenylphosphonate as substrate after 60 minsMore data for this Ligand-Target Pair
TargetTyrosyl-DNA phosphodiesterase 2(Homo sapiens (Human))
University Of Manchester
Curated by ChEMBL
University Of Manchester
Curated by ChEMBL
Affinity DataIC50: 7.10E+4nMpH: 7.5Assay Description:TDP2 reactions were carried out as described previously23 with the following modifications. A 18-mer single-stranded oligonucleotide DNA substrate (&...More data for this Ligand-Target Pair
Affinity DataIC50: >3.45E+5nMAssay Description:Inhibition of rat brain protein kinase CMore data for this Ligand-Target Pair
Affinity DataIC50: >1.11E+5nMpH: 7.5Assay Description:TDP2 reactions were carried out as described previously23 with the following modifications. A 18-mer single-stranded oligonucleotide DNA substrate (&...More data for this Ligand-Target Pair
TargetTyrosyl-DNA phosphodiesterase 2 [E242G,S244A,Q278R,I281T,K282R,R316G,P318T,Y321C](Mus musculus (Mouse))
National Institutes of Health
National Institutes of Health
Affinity DataIC50: >1.11E+5nMpH: 7.5Assay Description:TDP2 reactions were carried out as described previously23 with the following modifications. A 18-mer single-stranded oligonucleotide DNA substrate (&...More data for this Ligand-Target Pair
TargetTyrosyl-DNA phosphodiesterase 2 [E242G,S244A,Q278R,I281T,K282R,R316G,P318T,Y321CH323L](Mus musculus (Mouse))
National Institutes of Health
National Institutes of Health
Affinity DataIC50: 7.10E+4nMpH: 7.5Assay Description:TDP2 reactions were carried out as described previously23 with the following modifications. A 18-mer single-stranded oligonucleotide DNA substrate (&...More data for this Ligand-Target Pair
TargetTyrosyl-DNA phosphodiesterase 2(Homo sapiens (Human))
University Of Manchester
Curated by ChEMBL
University Of Manchester
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMpH: 7.5Assay Description:Ten-million cells (1 x 107), either human, chicken DT40 wild type, or knockout for TDP2 and complemented with human TDP2, were collected, washed, and...More data for this Ligand-Target Pair