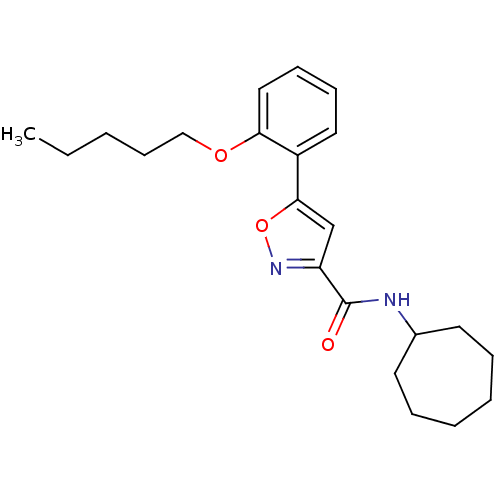
BDBM50448278 CHEMBL2414035
SMILES CCCCCOc1ccccc1-c1cc(no1)C(=O)NC1CCCCCC1
InChI Key InChIKey=PXNXWXIDTURTAX-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50448278
Found 4 hits for monomerid = 50448278
Affinity DataKi: 61nMAssay Description:Displacement of [3H]-CP55,940 from human CB2 receptor expressed in CHO membranes after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: >3.00E+3nMAssay Description:Displacement of [3H]-SR141716A from human CB1 receptor expressed in CHO membranes after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 5.43E+3nMAssay Description:Inhibition of human recombinant FAAH expressed in Escherichia coli assessed as hydrolysis of [3H]-AEAMore data for this Ligand-Target Pair
Affinity DataEC50: 11nMAssay Description:Agonist activity at human CB2 receptor expressed in CHO membranes assessed as [35S]-GTPgammaS binding after 1 hrMore data for this Ligand-Target Pair