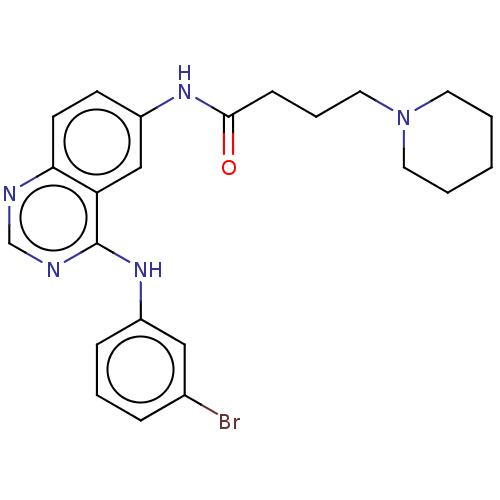
BDBM50493385 CHEMBL2425735
SMILES Brc1cccc(Nc2ncnc3ccc(NC(=O)CCCN4CCCCC4)cc23)c1
InChI Key InChIKey=KLBYVSOKDTXAPK-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50493385
Found 3 hits for monomerid = 50493385
Affinity DataIC50: 5.70nMAssay Description:Inhibition of wild type EGFR autophosphorylation in human A549 cells incubated for 1 hr followed by compound washout measured at 1 hr by Western blot...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of wild type EGFR autophosphorylation in human A549 cells incubated for 1 hr followed by compound washout measured after 8 hrs by Western ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.630nMAssay Description:Inhibition of wild type human EGFR tyrosine kinase assessed as Ulight-CAGAGAIETDKEYYTVKD phosphorylation after 15 mins by time-resolved fluorometryMore data for this Ligand-Target Pair