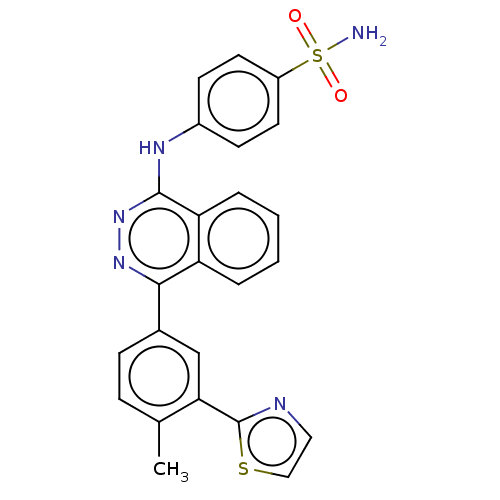
BDBM50597642 CHEMBL5182554
SMILES Cc1ccc(cc1-c1nccs1)-c1nnc(Nc2ccc(cc2)S(N)(=O)=O)c2ccccc12
InChI Key InChIKey=QOLXCXJVCNMSMN-UHFFFAOYSA-N
Data 3 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50597642
Found 3 hits for monomerid = 50597642
Affinity DataKi: 20nMAssay Description:Inhibition of human carbonic anhydrase 9 incubated for 15 mins prior to testing by phenol red based stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 74nMAssay Description:Inhibition of human carbonic anhydrase 2 incubated for 15 mins prior to testing by phenol red based stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
TargetEctonucleotide pyrophosphatase/phosphodiesterase family member 3(Homo sapiens (Human))
University Of Bonn
Curated by ChEMBL
University Of Bonn
Curated by ChEMBL
Affinity DataKi: 2.01E+4nMAssay Description:Inhibition of recombinant human soluble NPP3 expressed in Sf9 insect cells using ATP as substrate measured after 4 hrs by capillary electrophoresisMore data for this Ligand-Target Pair