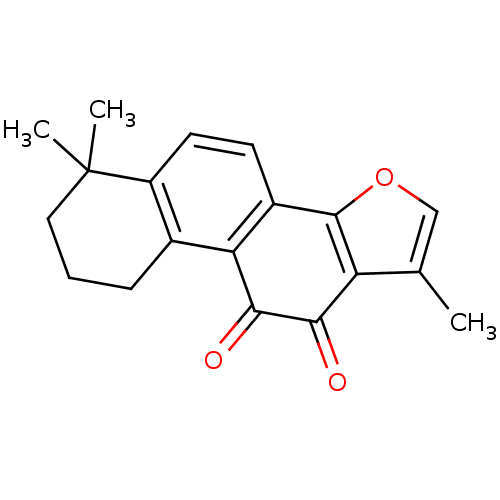
BDBM83922 1,6,6-trimethyl-8,9-dihydro-7H-naphtho[1,2-g][1]benzofuran-10,11-dione::1,6,6-trimethyl-8,9-dihydro-7H-naphtho[1,2-g]benzofuran-10,11-dione::1,6,6-trimethyl-8,9-dihydro-7H-naphtho[1,2-g]benzofuran-10,11-quinone::MLS001048863::SMR000387068::acs.jmedchem.1c00409_ST.743::cid_164676::med.21724, Compound 173
SMILES Cc1coc-2c1C(=O)C(=O)c1c3CCCC(C)(C)c3ccc-21
InChI Key InChIKey=HYXITZLLTYIPOF-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 83922
Found 2 hits for monomerid = 83922
TargetEndothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha(Homo sapiens (Human))
Korean Research Institute Of Biosciences And Biotechnology
Curated by ChEMBL
Korean Research Institute Of Biosciences And Biotechnology
Curated by ChEMBL
Affinity DataIC50: 7.53E+3nMAssay Description:Inhibition of HIF1 activation in human AGS cells assessed as inhibition of hypoxia-induced luciferase expression after 16 hrs by reporter assayMore data for this Ligand-Target Pair
TargetEndothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha(Homo sapiens (Human))
Korean Research Institute Of Biosciences And Biotechnology
Curated by ChEMBL
Korean Research Institute Of Biosciences And Biotechnology
Curated by ChEMBL
Affinity DataIC50: 6.18E+3nMAssay Description:Inhibition of HIF1 activation in human Hep3B cells assessed as inhibition of hypoxia-induced luciferase expression after 16 hrs by reporter assayMore data for this Ligand-Target Pair