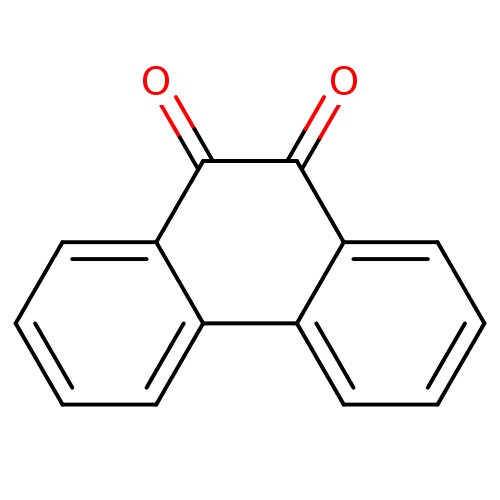
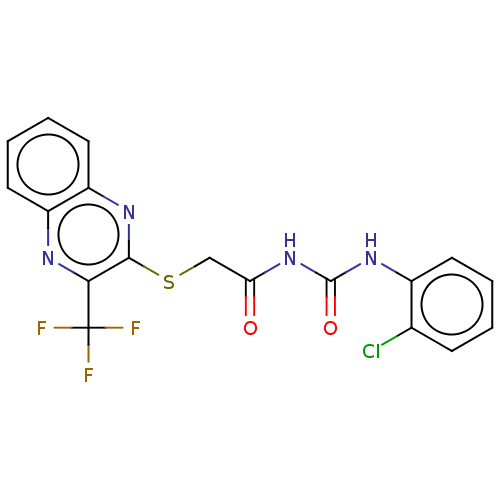
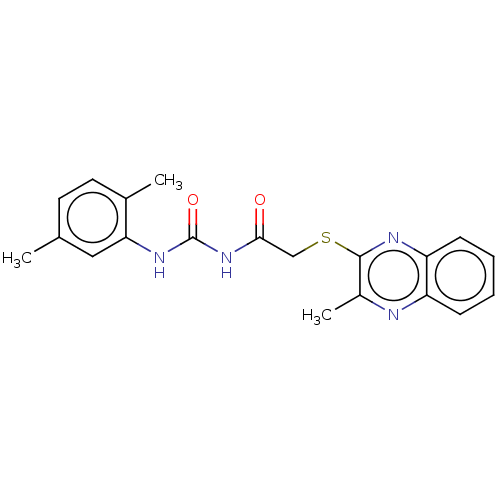
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 300nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 300nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 400nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
Affinity DataKi: 572nMAssay Description:Inhibition of Clostridium botulinum BoNT/A light chain assessed as inhibition of SNAP-25 (187-203) substrate hydrolysis by RP-HPLC-based assay in pre...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 700nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 700nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
Affinity DataKi: 900nMAssay Description:Inhibition of Clostridium botulinum BoNT/A light chain assessed as inhibition of SNAP-25 (187-203) substrate hydrolysis by RP-HPLC-based assay in pre...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
Affinity DataKi: 2.12E+3nMAssay Description:Inhibition of Clostridium botulinum BoNT/A light chain assessed as inhibition of SNAP-25 (187-203) substrate hydrolysis by RP-HPLC-based assay in pre...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 3.00E+3nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 3.00E+3nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 4.00E+3nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 6.00E+3nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 8.00E+3nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
Affinity DataKi: 8.52E+3nMAssay Description:Inhibition of Clostridium botulinum BoNT/A light chain assessed as inhibition of SNAP-25 (187-203) substrate hydrolysis by RP-HPLC-based assay in pre...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 1.00E+4nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 3.00E+4nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 6.00E+4nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 6.00E+4nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: >3.00E+5nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase C(Homo sapiens (Human))
United States Army Medical Research Institute of Infectious Diseases
United States Army Medical Research Institute of Infectious Diseases
Affinity DataIC50: 300nMAssay Description:Protein phosphatases were purchased from Upstate Biotechnology (Lake Placid, NY).More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase C(Homo sapiens (Human))
United States Army Medical Research Institute of Infectious Diseases
United States Army Medical Research Institute of Infectious Diseases
Affinity DataIC50: 400nMAssay Description:Protein phosphatases were purchased from Upstate Biotechnology (Lake Placid, NY).More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A2 [1-425](Clostridium botulinum)
National Cancer Institute At Frederick
National Cancer Institute At Frederick
Affinity DataIC50: 3.20E+3nMpH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase C(Homo sapiens (Human))
United States Army Medical Research Institute of Infectious Diseases
United States Army Medical Research Institute of Infectious Diseases
Affinity DataIC50: 6.00E+3nMAssay Description:Protein phosphatases were purchased from Upstate Biotechnology (Lake Placid, NY).More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A2 [1-425](Clostridium botulinum)
National Cancer Institute At Frederick
National Cancer Institute At Frederick
Affinity DataIC50: 7.00E+3nMpH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A2 [1-425](Clostridium botulinum)
National Cancer Institute At Frederick
National Cancer Institute At Frederick
Affinity DataIC50: 1.00E+4nMpH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A2 [1-425](Clostridium botulinum)
National Cancer Institute At Frederick
National Cancer Institute At Frederick
Affinity DataIC50: 1.70E+4nMpH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
Affinity DataIC50: >3.30E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) using midazolam as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >3.30E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) using midazolam as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >3.30E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) using midazolam as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >3.30E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) using midazolam as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >3.30E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) using midazolam as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >3.30E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) using midazolam as substrateMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A2 [1-425](Clostridium botulinum)
National Cancer Institute At Frederick
National Cancer Institute At Frederick
Affinity DatapH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A2 [1-425](Clostridium botulinum)
National Cancer Institute At Frederick
National Cancer Institute At Frederick
Affinity DatapH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A2 [1-425](Clostridium botulinum)
National Cancer Institute At Frederick
National Cancer Institute At Frederick
Affinity DatapH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A2 [1-425](Clostridium botulinum)
National Cancer Institute At Frederick
National Cancer Institute At Frederick
Affinity DatapH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A2 [1-425](Clostridium botulinum)
National Cancer Institute At Frederick
National Cancer Institute At Frederick
Affinity DatapH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A2 [1-425](Clostridium botulinum)
National Cancer Institute At Frederick
National Cancer Institute At Frederick
Affinity DatapH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A2 [1-425](Clostridium botulinum)
National Cancer Institute At Frederick
National Cancer Institute At Frederick
Affinity DatapH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair