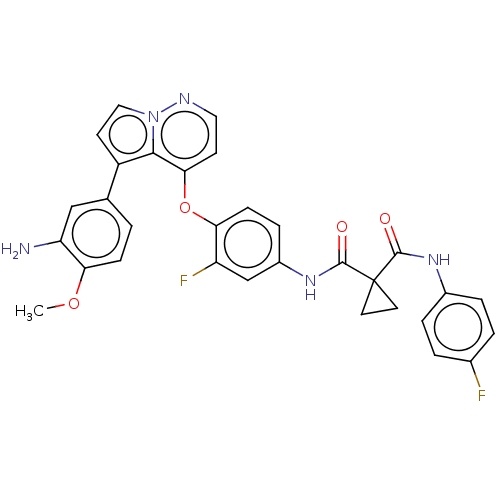
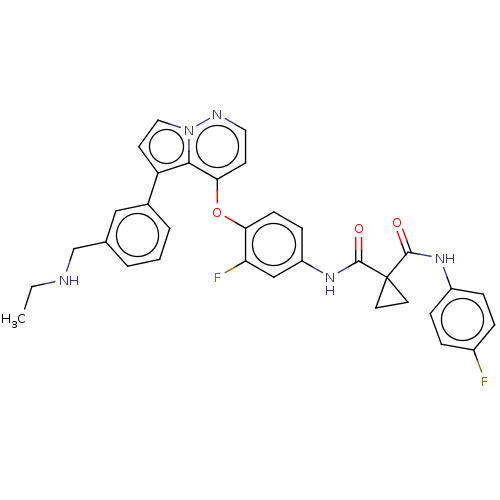
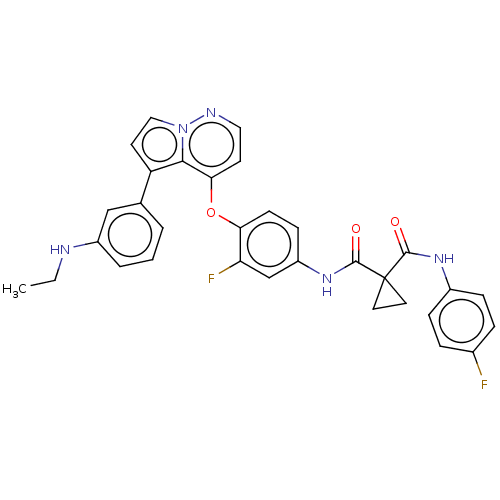
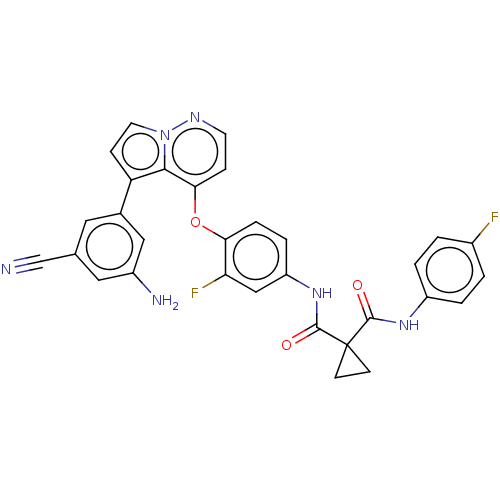
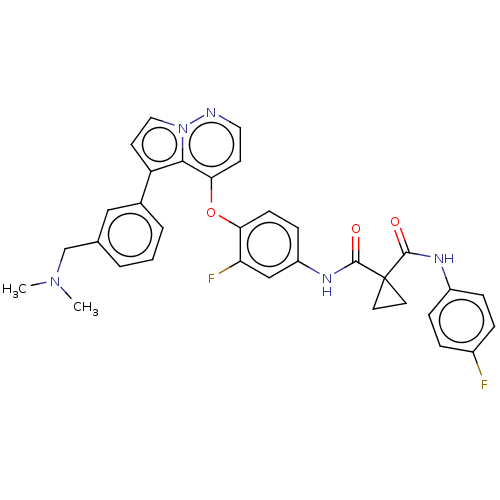
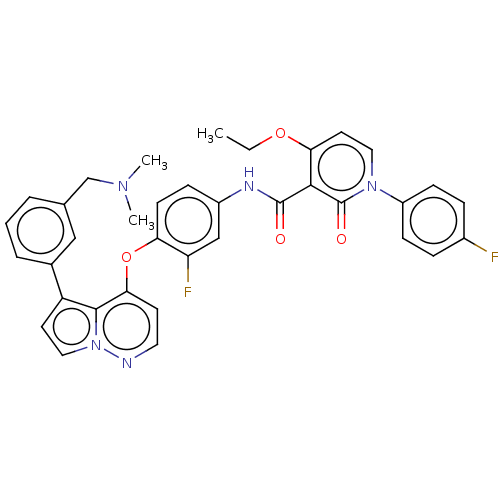
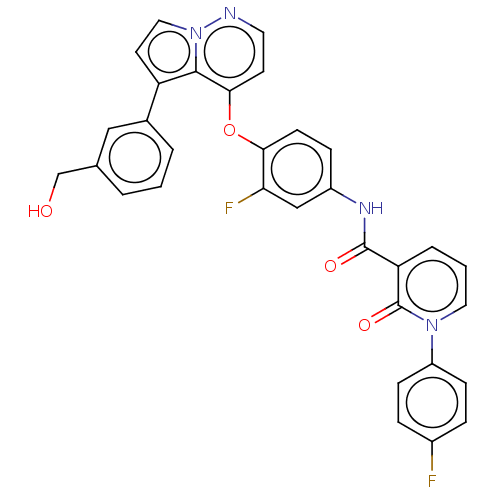
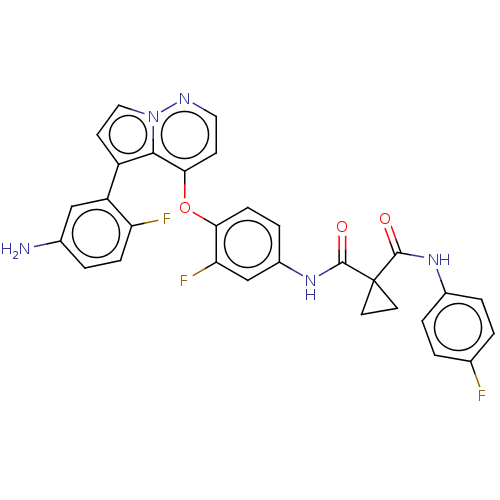
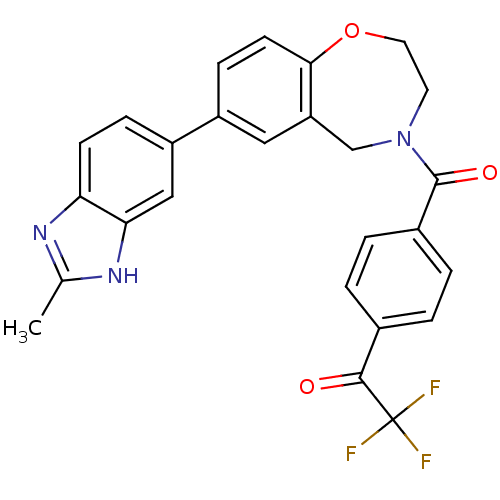
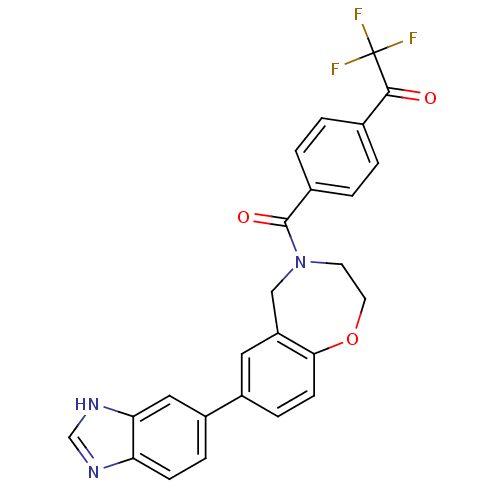
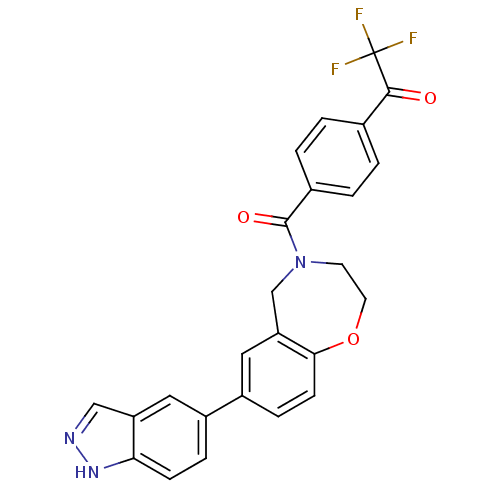
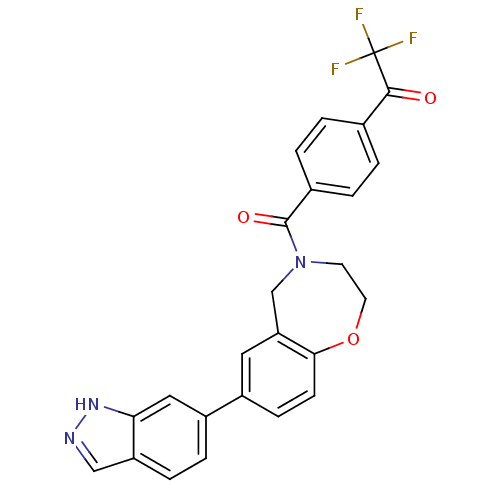
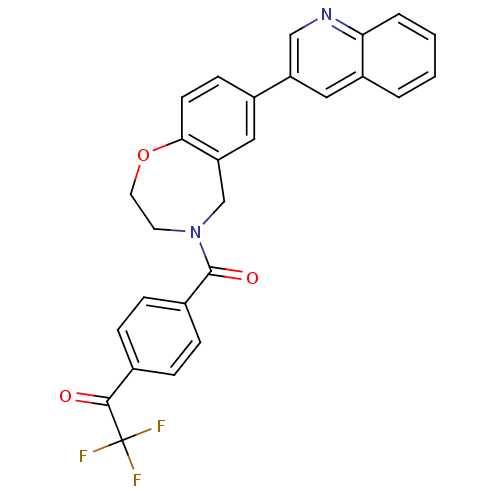
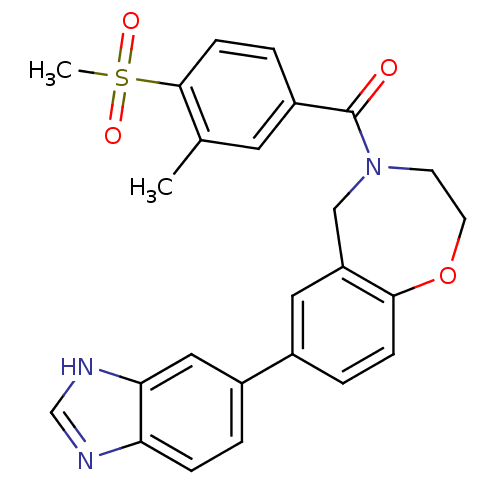
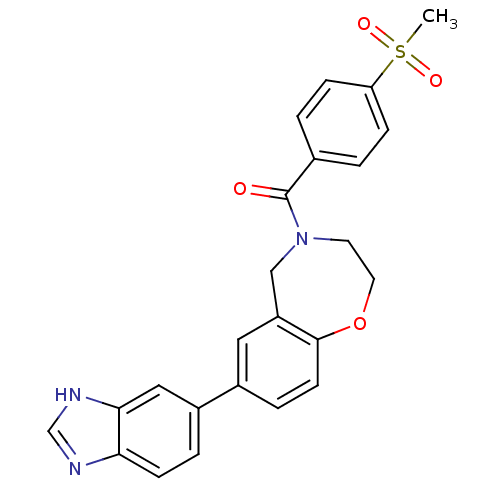
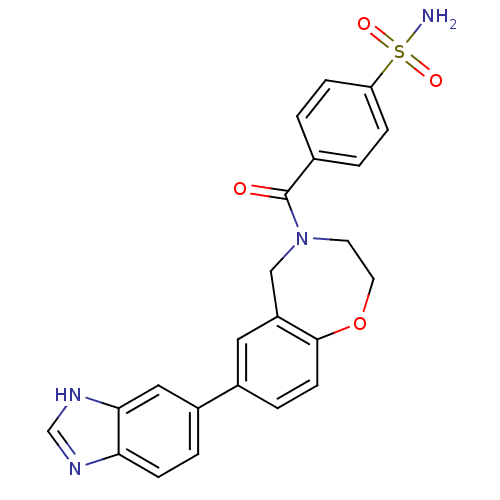
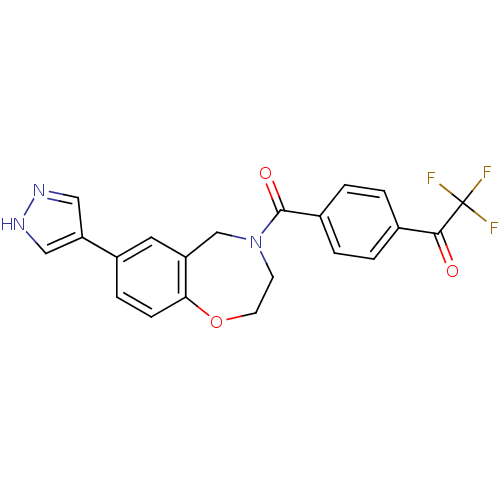
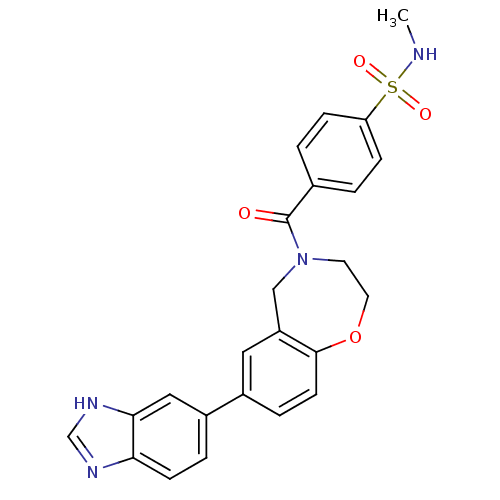
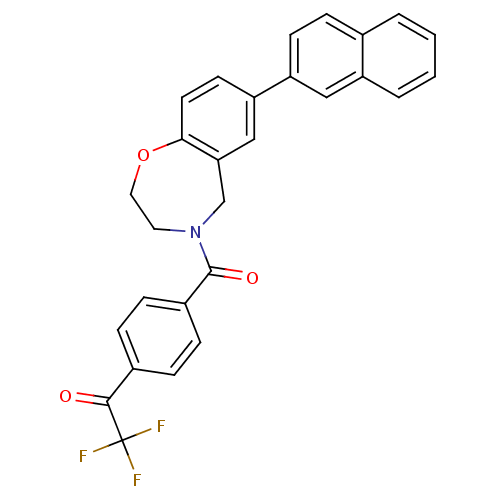
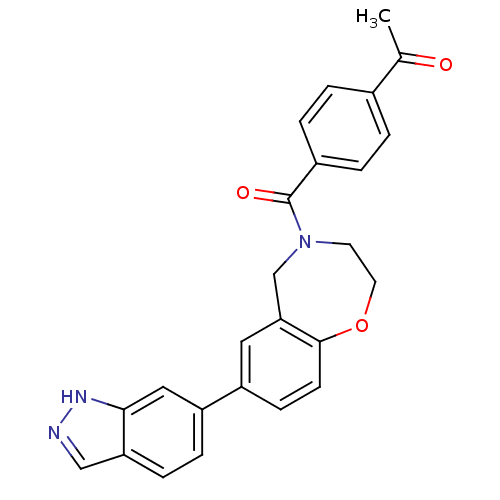
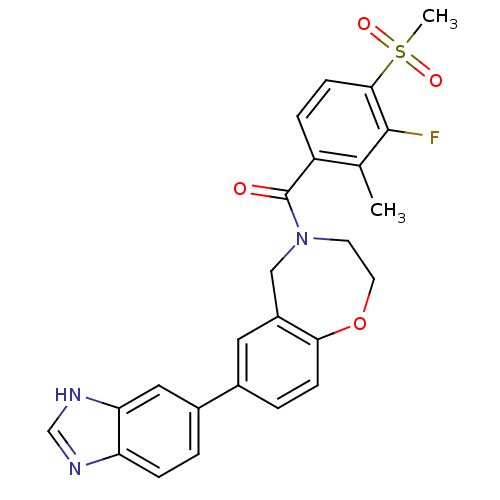
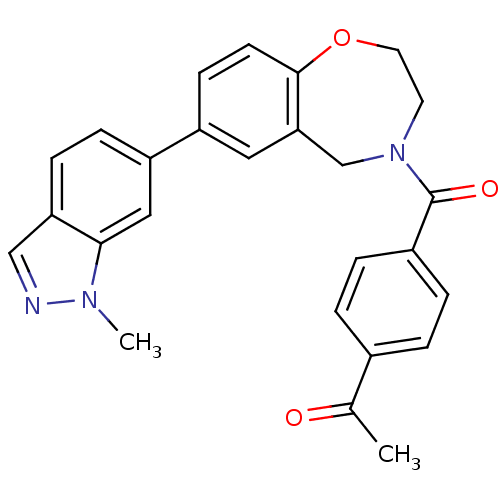
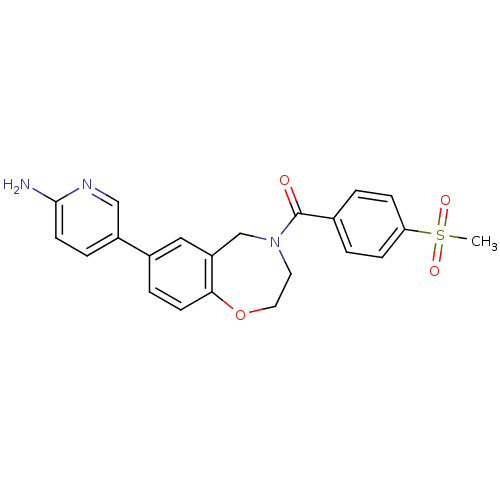
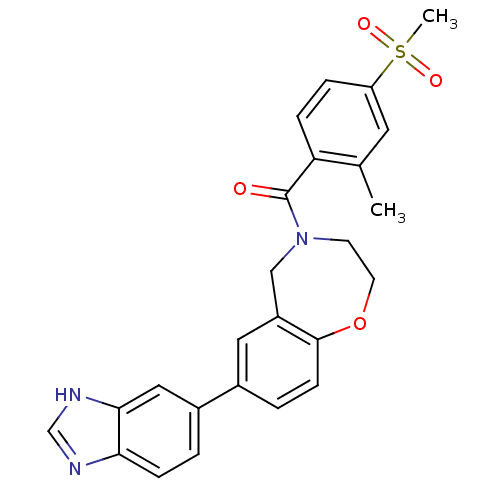
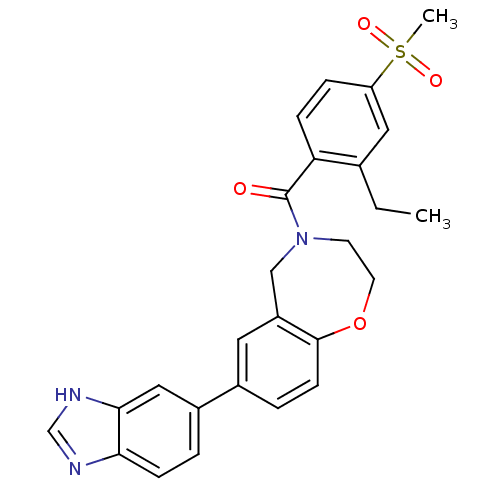
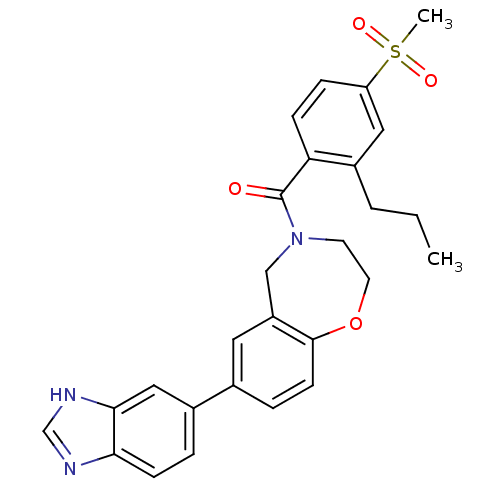
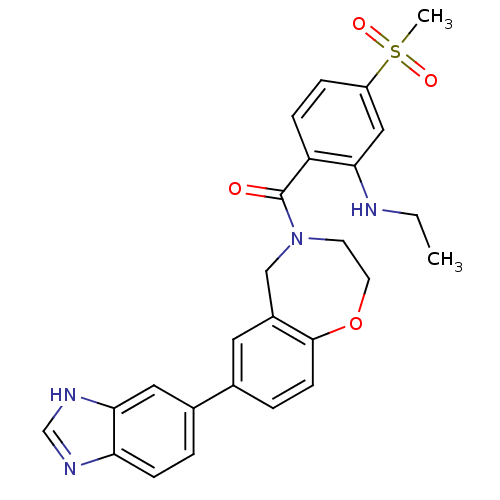
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 89nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 89nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 137nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 238nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 259nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 398nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 459nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 670nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 693nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 705nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 763nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 1.05E+3nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 1.09E+3nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 1.09E+3nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 1.13E+3nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 1.46E+3nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 2.17E+3nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 2.31E+3nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 2.34E+3nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 2.45E+3nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 2.90E+3nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: >3.00E+3nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: >3.00E+3nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: >3.00E+3nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: >3.00E+3nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assayMore data for this Ligand-Target Pair