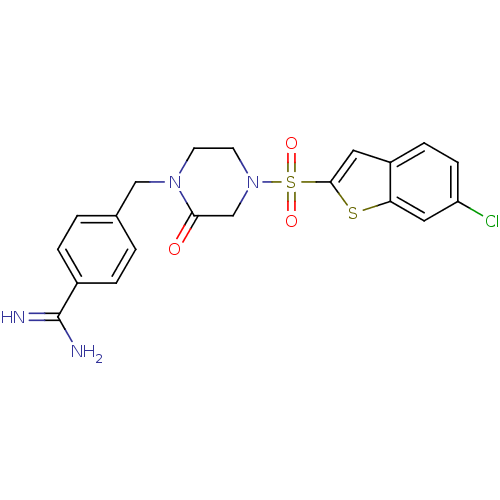
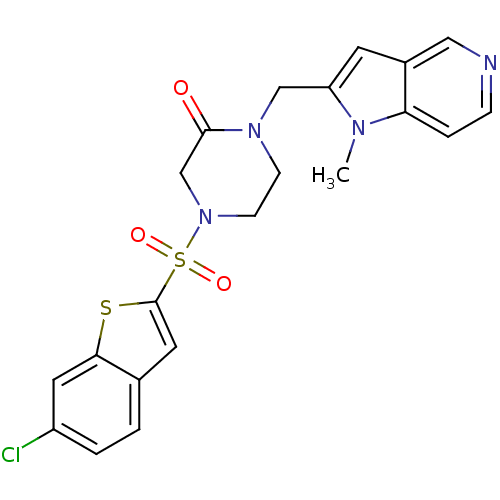
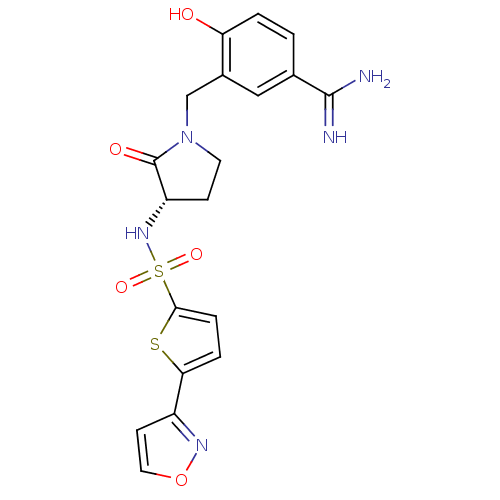
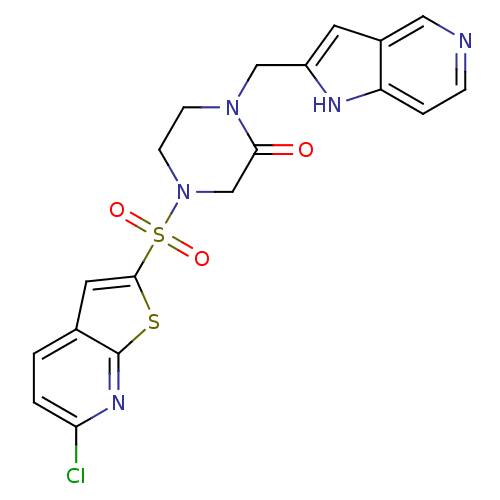
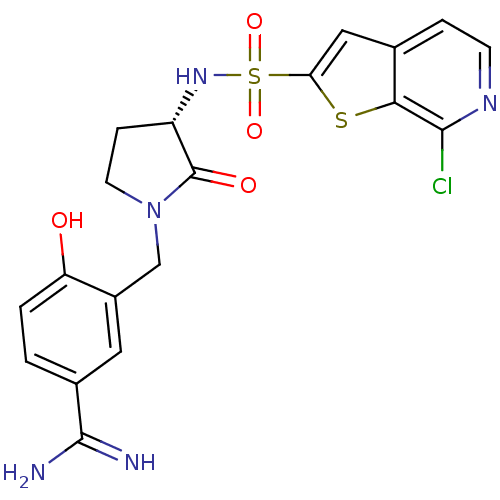
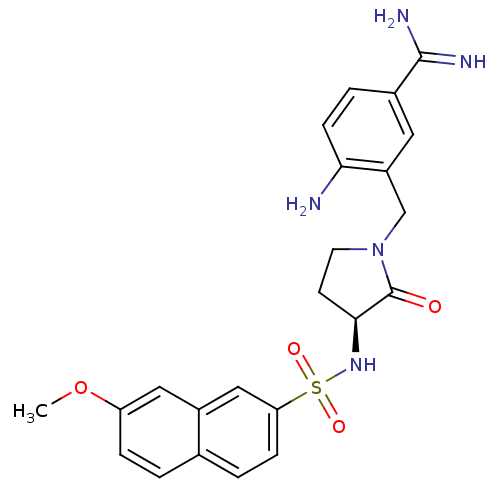
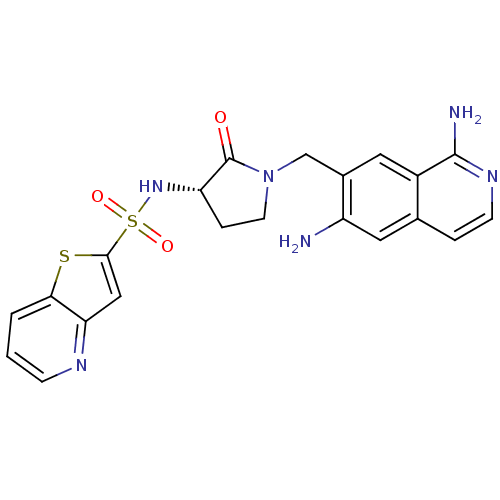
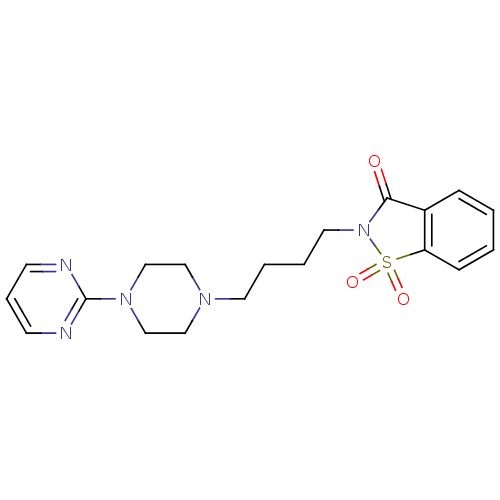
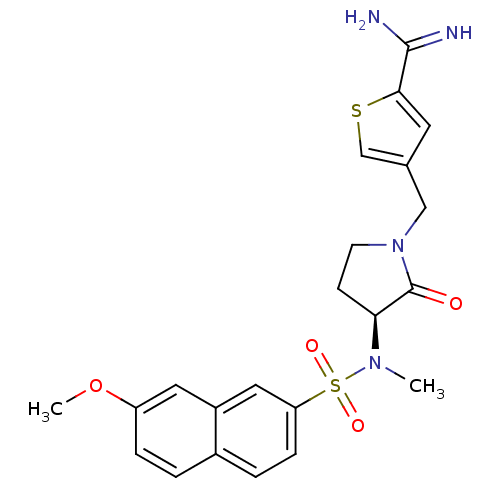
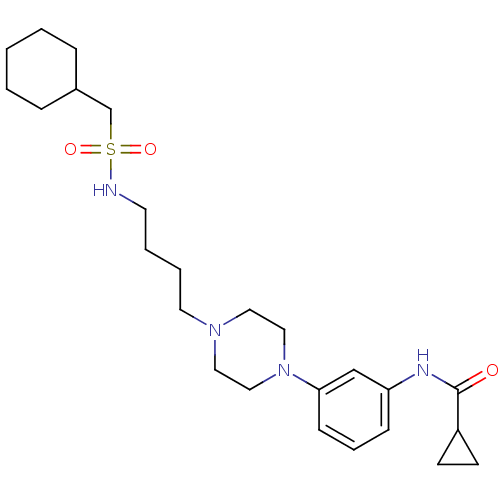
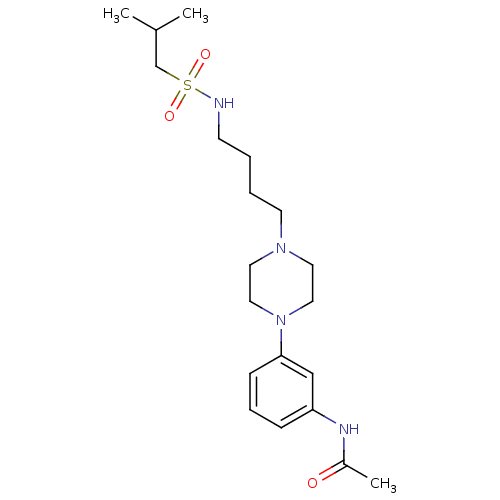
Affinity DataKi: 0.0470nMAssay Description:Compound was evaluated for the inhibition of human Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nM ΔG°: -51.7kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:Binding affinity against serine protease factor Xa (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:Binding affinity (in vitro) of the compound towards human Coagulation factor X was determined at 10 mg/kg peroral doseMore data for this Ligand-Target Pair
Affinity DataKi: 0.900nM ΔG°: -51.1kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 0.900nM ΔG°: -51.1kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Inhibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Inhibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Displacement of [3H]8-OH-DPAT from human 5HT1A receptor in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Binding affinity (in vitro) of the compound towards human Coagulation factor X was determined at 5 mg/kg peroral doseMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nM ΔG°: -50.6kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Displacement of [3H]8-OH-DPAT from human 5HT1A receptor in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.30nM ΔG°: -50.2kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Binding affinity against serine protease factor Xa (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Binding affinity against serine protease Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Binding affinity against serine protease factor Xa (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Binding affinity (in vitro) of the compound towards human Coagulation factor X was determined at 10 mg/kg peroral doseMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Binding affinity against serine protease factor Xa (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Inhibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Binding affinity against serine protease Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:In vitro binding affinity for human Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 3nM ΔG°: -48.2kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:In vitro binding affinity for human Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Binding affinity against serine protease factor Xa (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 3nM ΔG°: -48.2kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:In vitro binding affinity for human Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:In vitro binding affinity for human Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 4nM ΔG°: -47.5kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Binding affinity against serine protease factor Xa (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Binding affinity against serine protease factor Xa (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 5.10nMAssay Description:Displacement of [3H]8-OH-DPAT from human 5HT1A receptor in HEK293 cellsMore data for this Ligand-Target Pair
TargetAlpha-1A/Alpha-1B/Alpha-1D adrenergic receptor(Rattus norvegicus (rat))
Predix Pharmaceuticals
Curated by ChEMBL
Predix Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 6nMAssay Description:Displacement of [3H]prazosin from adrenergic alpha-1 receptor in rat cerebral cortex cellsMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:In vitro evaluation of inhibitory activity against Coagulation factor X in prothrombinase complexMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 6nM ΔG°: -46.5kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:In vitro evaluation of inhibitory activity against Coagulation factor X in prothrombinase complexMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Displacement of [3H]8-OH-DPAT from human 5HT1A receptor in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 6.80nMAssay Description:Displacement of [3H]8-OH-DPAT from human 5HT1A receptor in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 6.90nM ΔG°: -46.1kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:In vitro evaluation of inhibitory activity against Coagulation factor X in prothrombinase complexMore data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:Compound was evaluated for the inhibition of human Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:In vitro binding affinity for human Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 8nM ΔG°: -45.7kJ/molepH: 7.5 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 8.90nMAssay Description:Displacement of [3H]8-OH-DPAT from human 5HT1A receptor in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 9.20nMAssay Description:Displacement of [3H]8-OH-DPAT from human 5HT1A receptor in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 9.70nMAssay Description:Displacement of [3H]8-OH-DPAT from human 5HT1A receptor in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Inhibition of Coagulation factor XaMore data for this Ligand-Target Pair




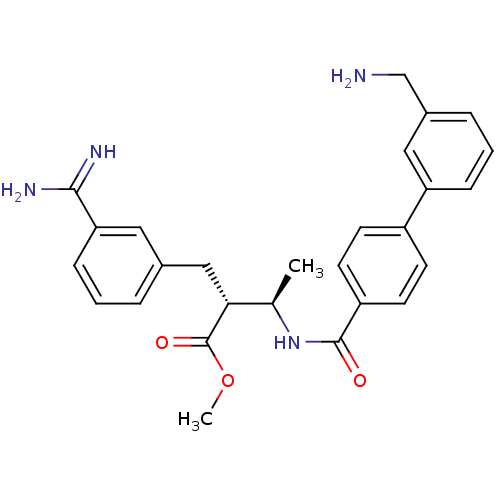
 3D Structure (crystal)
3D Structure (crystal)