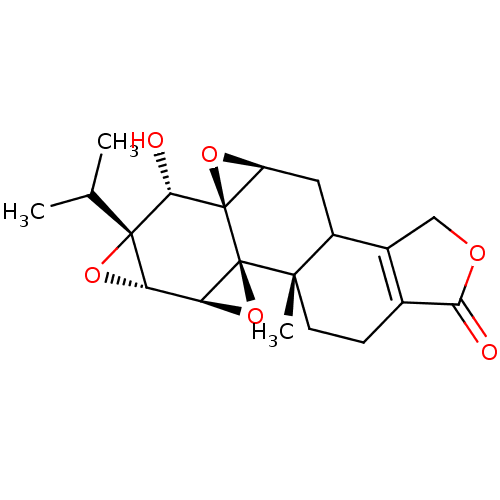
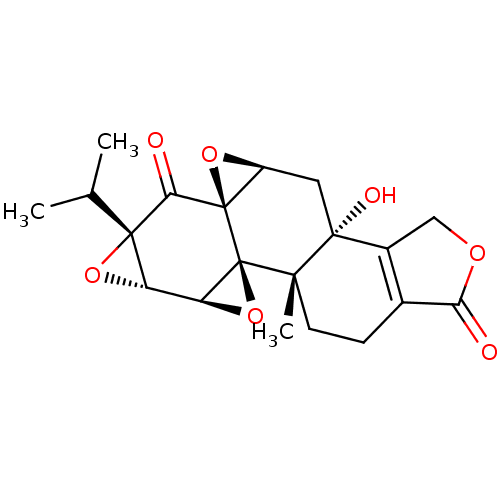
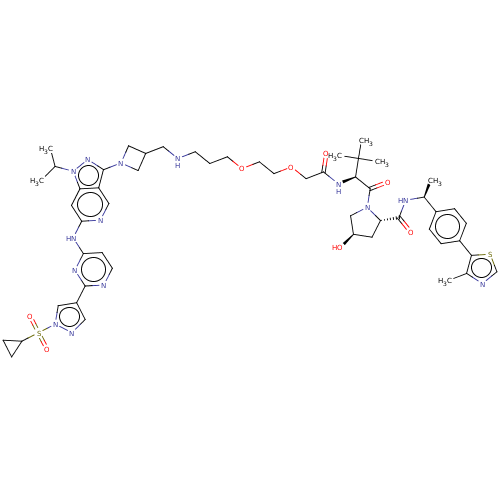
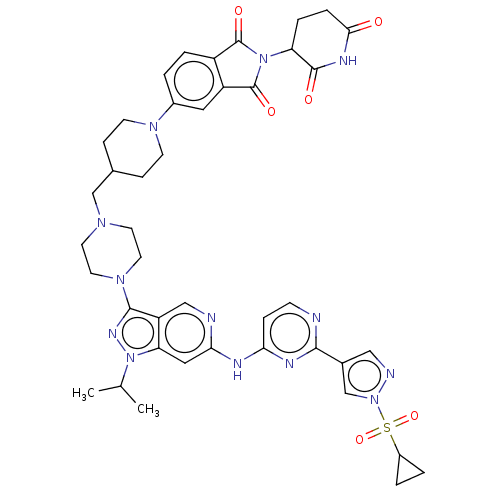
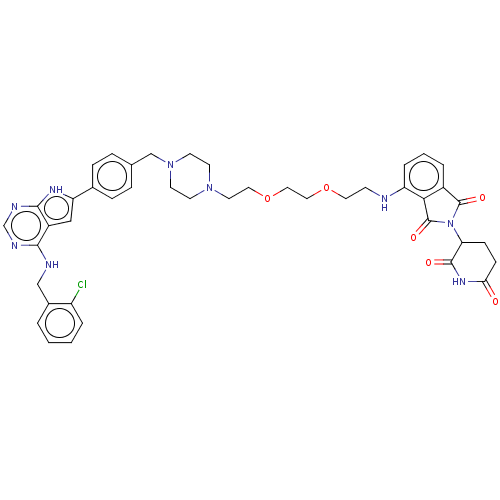
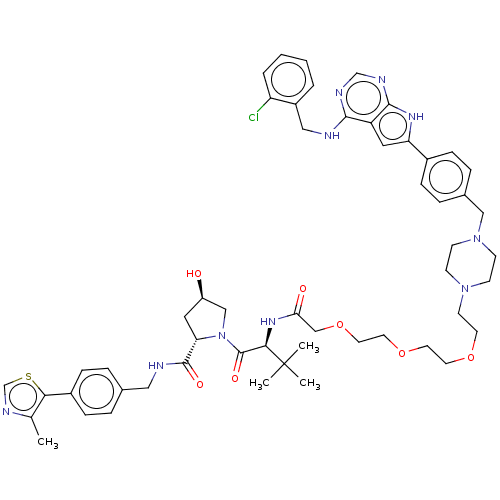
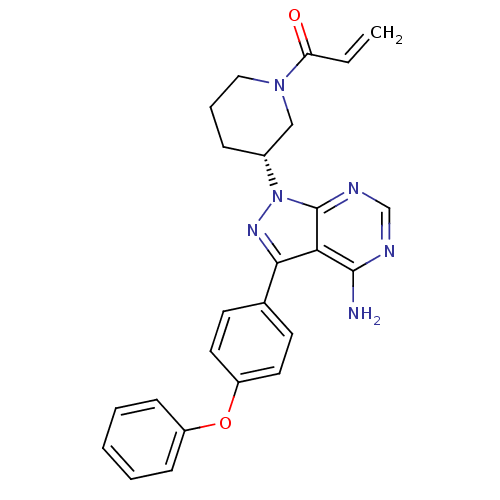
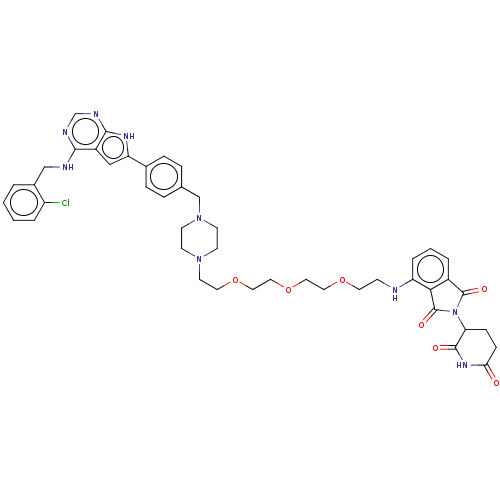
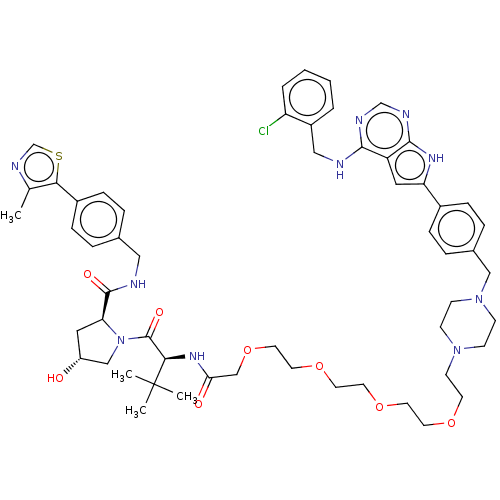
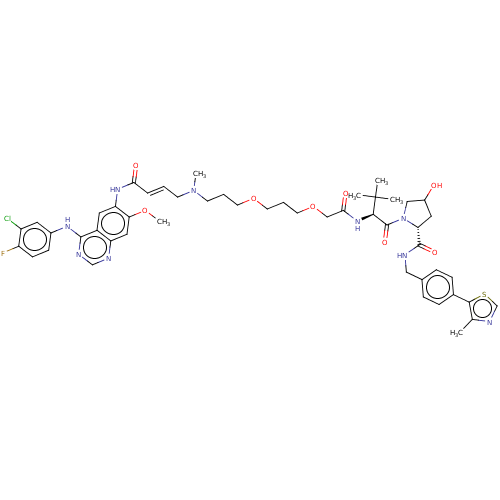
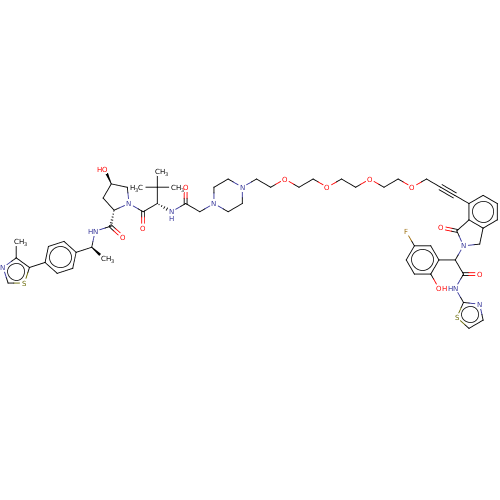
Affinity DataKi: 8.50E+4nM ΔG°: -23.2kJ/molepH: 7.4 T: 2°CAssay Description:Kinetic enzyme assays were performed in a 384-well, white plates (Nunc) on a Wallac Victor 2 plate reader using human DCTPP1 and triptolide and analo...More data for this Ligand-Target Pair
Affinity DataKi: 1.35E+5nM ΔG°: -22.1kJ/molepH: 7.4 T: 2°CAssay Description:Kinetic enzyme assays were performed in a 384-well, white plates (Nunc) on a Wallac Victor 2 plate reader using human DCTPP1 and triptolide and analo...More data for this Ligand-Target Pair
Affinity DataKi: 1.68E+5nM ΔG°: -21.5kJ/molepH: 7.4 T: 2°CAssay Description:Kinetic enzyme assays were performed in a 384-well, white plates (Nunc) on a Wallac Victor 2 plate reader using human DCTPP1 and triptolide and analo...More data for this Ligand-Target Pair
Affinity DataKi: 1.94E+5nM ΔG°: -21.2kJ/molepH: 7.4 T: 2°CAssay Description:Kinetic enzyme assays were performed in a 384-well, white plates (Nunc) on a Wallac Victor 2 plate reader using human DCTPP1 and triptolide and analo...More data for this Ligand-Target Pair
Affinity DataKi: 3.56E+5nM ΔG°: -19.7kJ/molepH: 7.4 T: 2°CAssay Description:Kinetic enzyme assays were performed in a 384-well, white plates (Nunc) on a Wallac Victor 2 plate reader using human DCTPP1 and triptolide and analo...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [1-18,20-745,747-749,751-1210](Homo sapiens (Human))
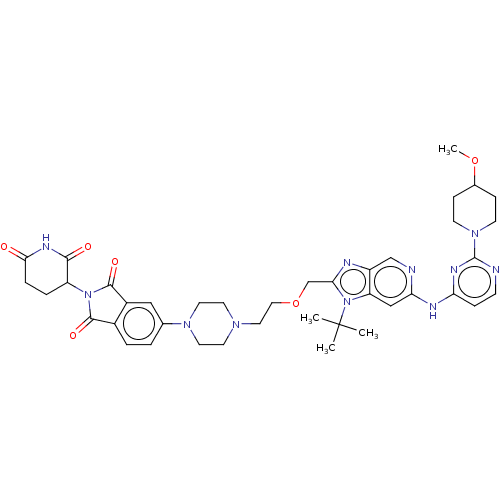
Arvinas Operations
US Patent
Arvinas Operations
US Patent
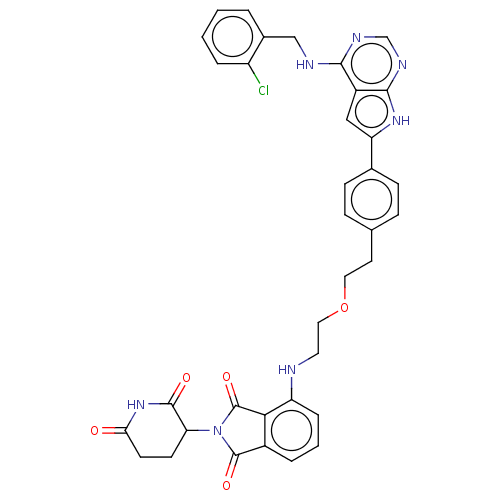
Affinity DataIC50: 0.140nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [L858R,T790M,C797S](Homo sapiens (Human))
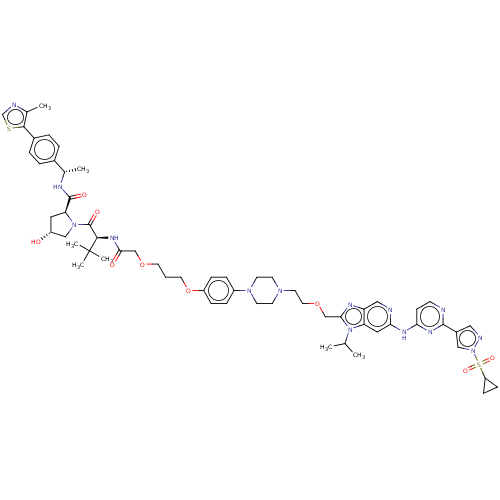
Arvinas Operations
US Patent
Arvinas Operations
US Patent
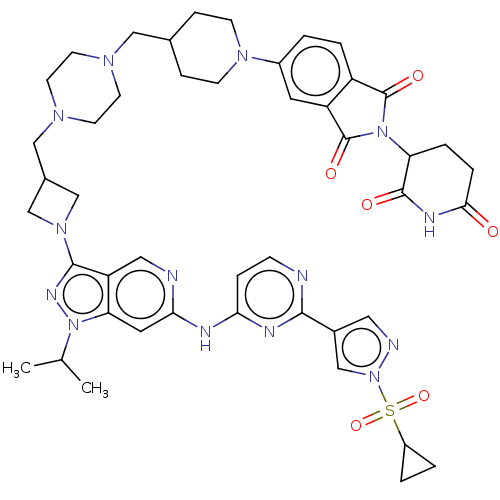
Affinity DataIC50: 0.260nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [L858R,T790M,C797S](Homo sapiens (Human))
Arvinas Operations
US Patent
Arvinas Operations
US Patent
Affinity DataIC50: 0.270nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [T790M,L858R](Homo sapiens (Human))
Arvinas Operations
US Patent
Arvinas Operations
US Patent
Affinity DataIC50: 0.290nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [1-18,20-745,747-749,751-1210,T790M](Homo sapiens (Human))
Arvinas Operations
US Patent
Arvinas Operations
US Patent
Affinity DataIC50: 0.300nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [L858R,T790M,C797S](Homo sapiens (Human))
Arvinas Operations
US Patent
Arvinas Operations
US Patent
Affinity DataIC50: 0.350nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [T790M,L858R](Homo sapiens (Human))
Arvinas Operations
US Patent
Arvinas Operations
US Patent
Affinity DataIC50: 0.670nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [L858R,T790M,C797S](Homo sapiens (Human))
Arvinas Operations
US Patent
Arvinas Operations
US Patent
Affinity DataIC50: 0.800nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [T790M,L858R](Homo sapiens (Human))
Arvinas Operations
US Patent
Arvinas Operations
US Patent
Affinity DataIC50: 0.910nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [1-18,20-745,747-749,751-1210](Homo sapiens (Human))
Arvinas Operations
US Patent
Arvinas Operations
US Patent
Affinity DataIC50: 1nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [1-18,20-745,747-749,751-1210](Homo sapiens (Human))
Arvinas Operations
US Patent
Arvinas Operations
US Patent
Affinity DataIC50: 1.30nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Inhibition of TBK1 (unknown origin)More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [L858R,T790M,C797S](Homo sapiens (Human))
Arvinas Operations
US Patent
Arvinas Operations
US Patent
Affinity DataIC50: 1.30nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [1-18,20-745,747-749,751-1210,T790M](Homo sapiens (Human))
Arvinas Operations
US Patent
Arvinas Operations
US Patent
Affinity DataIC50: 1.60nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [1-18,20-745,747-749,751-1210](Homo sapiens (Human))
Arvinas Operations
US Patent
Arvinas Operations
US Patent
Affinity DataIC50: 1.70nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [1-18,20-745,747-749,751-1210](Homo sapiens (Human))
Arvinas Operations
US Patent
Arvinas Operations
US Patent
Affinity DataIC50: 1.90nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [1-18,20-745,747-749,751-1210,T790M](Homo sapiens (Human))
Arvinas Operations
US Patent
Arvinas Operations
US Patent
Affinity DataIC50: 2.10nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [1-18,20-745,747-749,751-1210](Homo sapiens (Human))
Arvinas Operations
US Patent
Arvinas Operations
US Patent
Affinity DataIC50: 2.10nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:Inhibition of BTK (unknown origin)More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [T790M,L858R](Homo sapiens (Human))
Arvinas Operations
US Patent
Arvinas Operations
US Patent
Affinity DataIC50: 2.30nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [1-18,20-745,747-749,751-1210](Homo sapiens (Human))
Arvinas Operations
US Patent
Arvinas Operations
US Patent
Affinity DataIC50: 2.90nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [1-18,20-745,747-749,751-1210](Homo sapiens (Human))
Arvinas Operations
US Patent
Arvinas Operations
US Patent
Affinity DataIC50: 4nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [1-18,20-745,747-749,751-1210](Homo sapiens (Human))
Arvinas Operations
US Patent
Arvinas Operations
US Patent
Affinity DataIC50: 4.10nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
Affinity DataIC50: 4.20nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [1-18,20-745,747-749,751-1210](Homo sapiens (Human))
Arvinas Operations
US Patent
Arvinas Operations
US Patent
Affinity DataIC50: 4.20nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
Affinity DataIC50: 4.70nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [1-18,20-745,747-749,751-1210,T790M](Homo sapiens (Human))
Arvinas Operations
US Patent
Arvinas Operations
US Patent
Affinity DataIC50: 4.90nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
Affinity DataIC50: 4.90nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [1-18,20-745,747-749,751-1210](Homo sapiens (Human))
Arvinas Operations
US Patent
Arvinas Operations
US Patent
Affinity DataIC50: 5.5nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
Affinity DataIC50: 7.20nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [1-18,20-745,747-749,751-1210](Homo sapiens (Human))
Arvinas Operations
US Patent
Arvinas Operations
US Patent
Affinity DataIC50: 7.5nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
Affinity DataIC50: 8.30nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
Affinity DataIC50: 8.40nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
TargetInhibitor of nuclear factor kappa-B kinase subunit epsilon(Homo sapiens (Human))
Arvinas
Curated by ChEMBL
Arvinas
Curated by ChEMBL
Affinity DataIC50: 8.70nMAssay Description:Inhibition of IKKepsilon (unknown origin)More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [T790M,L858R](Homo sapiens (Human))
Arvinas Operations
US Patent
Arvinas Operations
US Patent
Affinity DataIC50: 9.80nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [L858R,T790M,C797S](Homo sapiens (Human))
Arvinas Operations
US Patent
Arvinas Operations
US Patent
Affinity DataIC50: 16nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor [L858R,T790M,C797S](Homo sapiens (Human))
Arvinas Operations
US Patent
Arvinas Operations
US Patent
Affinity DataIC50: 16nMAssay Description:All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A...More data for this Ligand-Target Pair

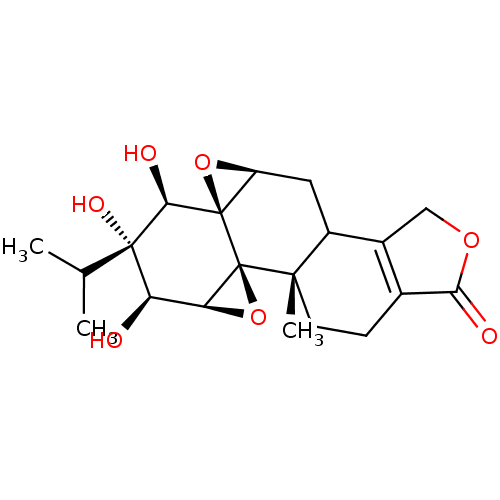
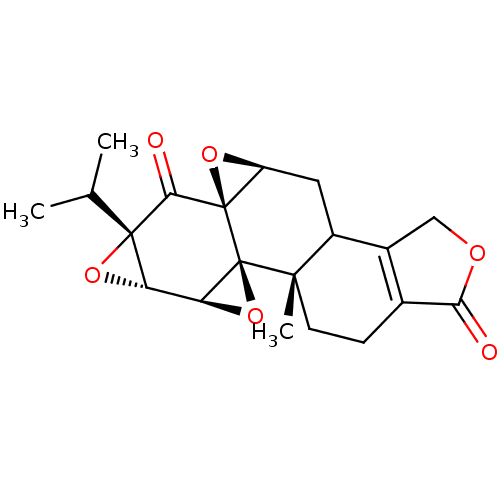
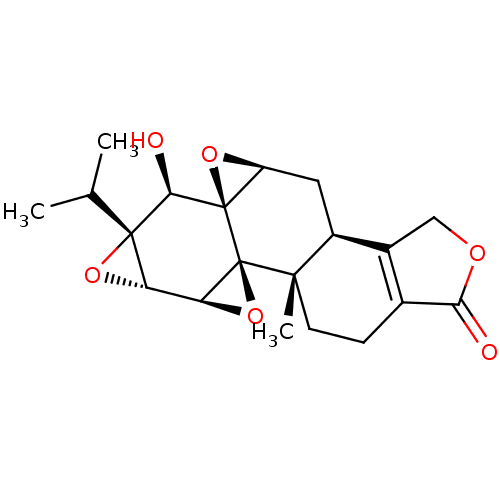
 3D Structure (crystal)
3D Structure (crystal)