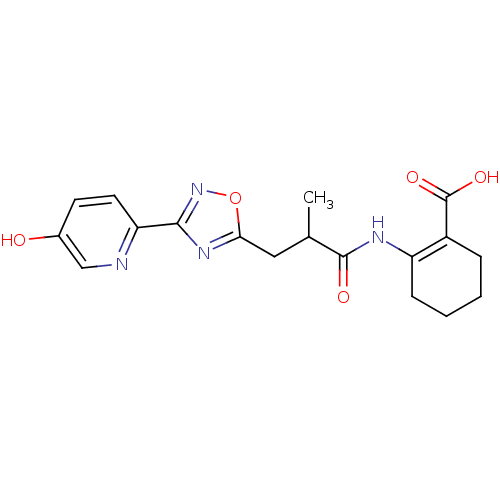
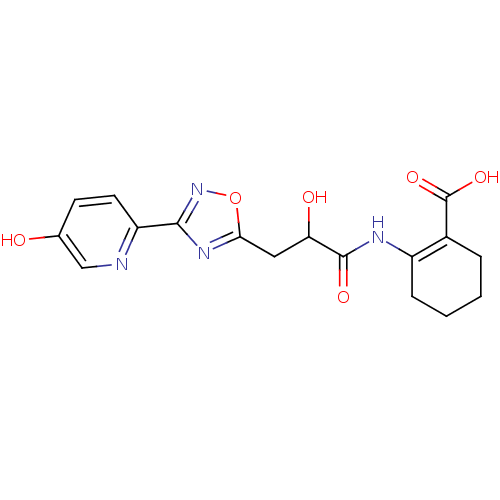
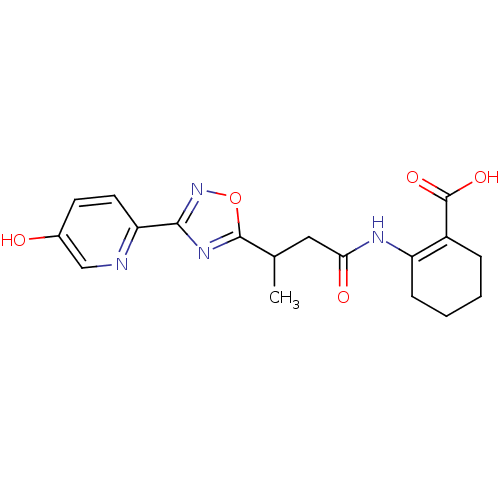
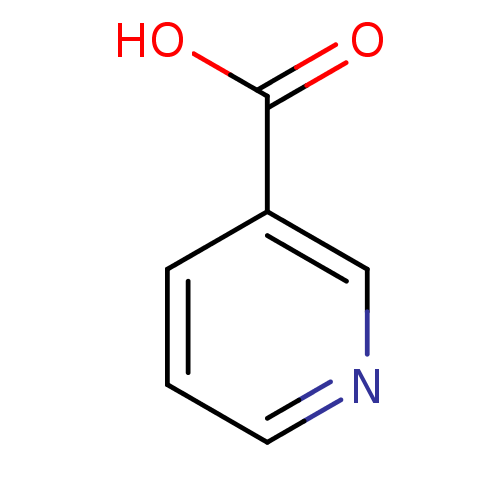
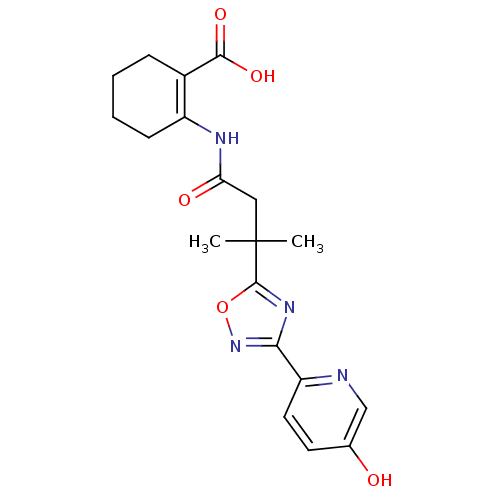
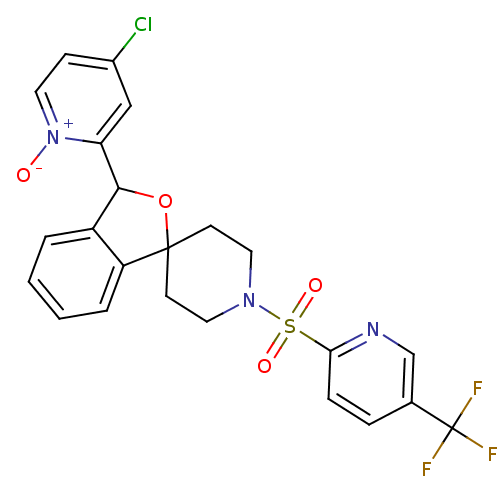
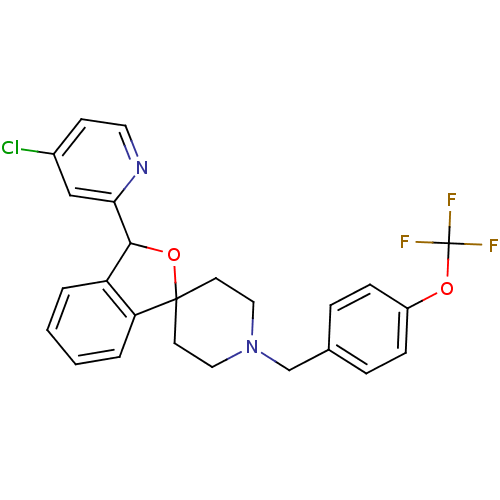
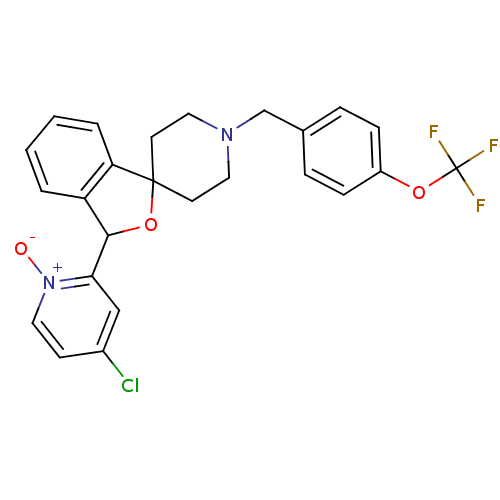
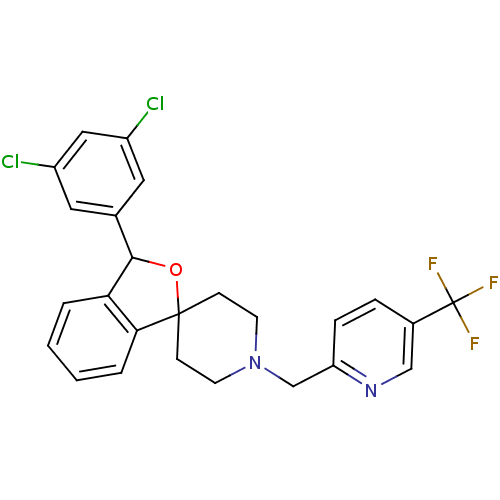
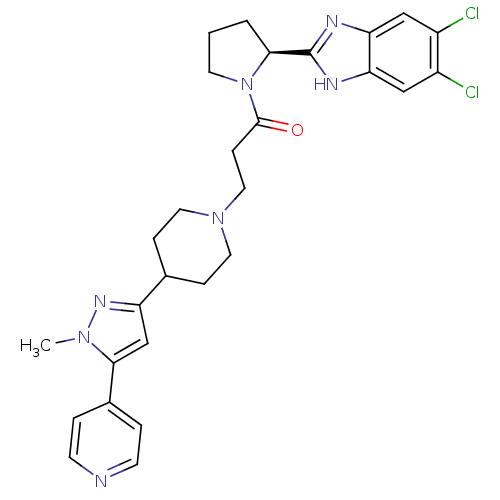
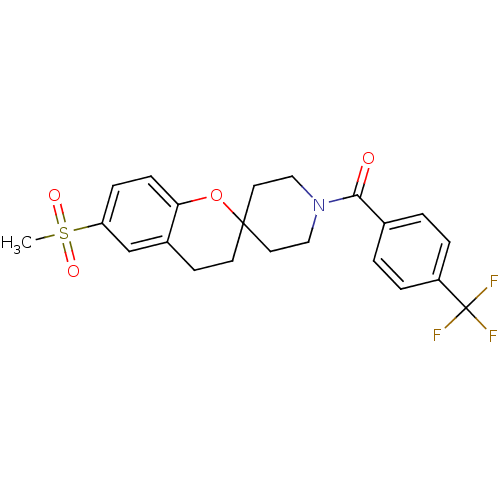
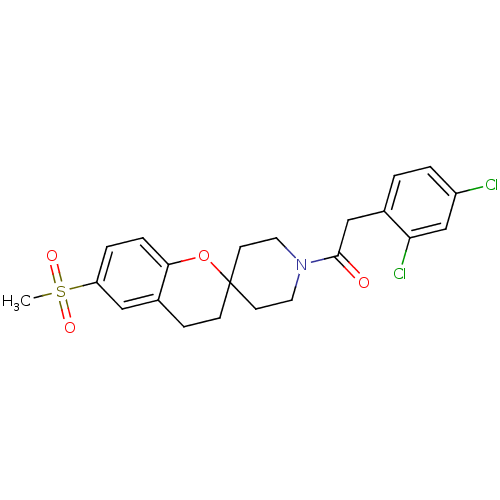
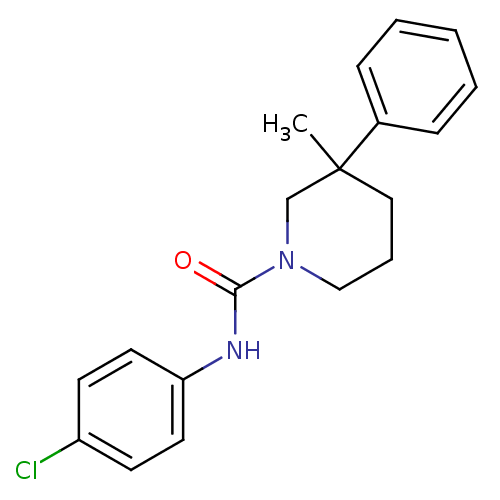
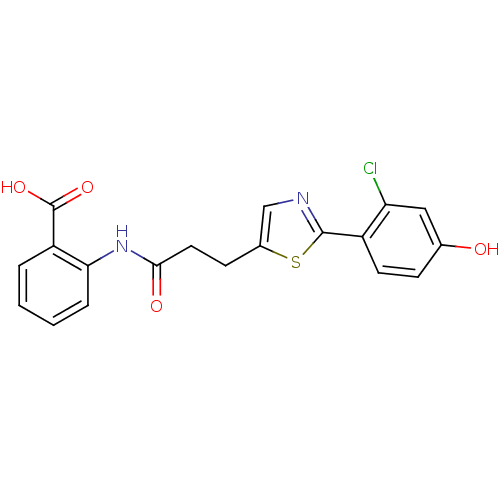
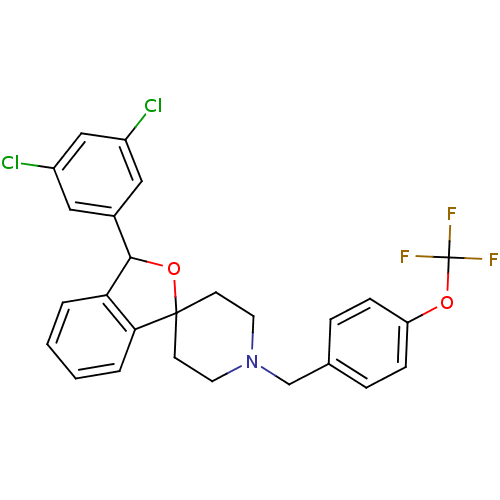
TargetHydroxycarboxylic acid receptor 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometryMore data for this Ligand-Target Pair
TargetHydroxycarboxylic acid receptor 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometryMore data for this Ligand-Target Pair
TargetHydroxycarboxylic acid receptor 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometryMore data for this Ligand-Target Pair
TargetHydroxycarboxylic acid receptor 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometryMore data for this Ligand-Target Pair
TargetHydroxycarboxylic acid receptor 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 8nMAssay Description:Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometryMore data for this Ligand-Target Pair
TargetHydroxycarboxylic acid receptor 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 8nMAssay Description:Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometryMore data for this Ligand-Target Pair
TargetHydroxycarboxylic acid receptor 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometryMore data for this Ligand-Target Pair
TargetHydroxycarboxylic acid receptor 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 22nMAssay Description:Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometryMore data for this Ligand-Target Pair
TargetHydroxycarboxylic acid receptor 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 29nMAssay Description:Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometryMore data for this Ligand-Target Pair
TargetHydroxycarboxylic acid receptor 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 82nMAssay Description:Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometryMore data for this Ligand-Target Pair
TargetHydroxycarboxylic acid receptor 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 104nMAssay Description:Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albuminMore data for this Ligand-Target Pair
TargetHydroxycarboxylic acid receptor 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 120nMAssay Description:Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albuminMore data for this Ligand-Target Pair
TargetHydroxycarboxylic acid receptor 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 130nMAssay Description:Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometryMore data for this Ligand-Target Pair
TargetHydroxycarboxylic acid receptor 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 150nMAssay Description:Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albuminMore data for this Ligand-Target Pair
TargetHydroxycarboxylic acid receptor 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 230nMAssay Description:Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albuminMore data for this Ligand-Target Pair
TargetHydroxycarboxylic acid receptor 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 240nMAssay Description:Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albuminMore data for this Ligand-Target Pair
TargetHydroxycarboxylic acid receptor 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 360nMAssay Description:Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometryMore data for this Ligand-Target Pair
TargetHydroxycarboxylic acid receptor 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 595nMAssay Description:Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albuminMore data for this Ligand-Target Pair
TargetHydroxycarboxylic acid receptor 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 660nMAssay Description:Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albuminMore data for this Ligand-Target Pair
TargetHydroxycarboxylic acid receptor 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 840nMAssay Description:Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albuminMore data for this Ligand-Target Pair
TargetHydroxycarboxylic acid receptor 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.08E+3nMAssay Description:Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albuminMore data for this Ligand-Target Pair
TargetHydroxycarboxylic acid receptor 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.10E+3nMAssay Description:Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albuminMore data for this Ligand-Target Pair
TargetHydroxycarboxylic acid receptor 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 9.13E+3nMAssay Description:Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albuminMore data for this Ligand-Target Pair
TargetHydroxycarboxylic acid receptor 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 2.47E+4nMAssay Description:Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albuminMore data for this Ligand-Target Pair
Affinity DataIC50: 0.110nMAssay Description:Inhibition of mouse PrCPMore data for this Ligand-Target Pair
Affinity DataIC50: 0.180nMAssay Description:Inhibition of mouse PrCPMore data for this Ligand-Target Pair
Affinity DataIC50: 0.310nMAssay Description:Inhibition of mouse PrCPMore data for this Ligand-Target Pair
Affinity DataIC50: 0.380nMAssay Description:Inhibition of mouse PrCPMore data for this Ligand-Target Pair
Affinity DataIC50: 0.390nMAssay Description:Inhibition of mouse PrCPMore data for this Ligand-Target Pair
Affinity DataIC50: 0.410nMAssay Description:Inhibition of mouse PrCPMore data for this Ligand-Target Pair
TargetLysosomal Pro-X carboxypeptidase(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.440nMAssay Description:Inhibition of human PrCPMore data for this Ligand-Target Pair
TargetLysosomal Pro-X carboxypeptidase(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.490nMAssay Description:Inhibition of human PrCPMore data for this Ligand-Target Pair
Affinity DataIC50: 0.530nMAssay Description:Inhibition of mouse PrCPMore data for this Ligand-Target Pair
Affinity DataIC50: 0.710nMAssay Description:Inhibition of mouse PrCPMore data for this Ligand-Target Pair
TargetLysosomal Pro-X carboxypeptidase(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.730nMAssay Description:Inhibition of human PrCPMore data for this Ligand-Target Pair
TargetLysosomal Pro-X carboxypeptidase(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.780nMAssay Description:Inhibition of human PrCPMore data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Inhibition of mouse PrCPMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: <1nMAssay Description:Inhibition of human soluble epoxide hydrolase in HEK293 cells assessed as DHET productionMore data for this Ligand-Target Pair
TargetHydroxycarboxylic acid receptor 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Displacement of [3H]niacin from human GPR109A expressed in CHO cellsMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: <1nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: <1nMAssay Description:Inhibition of human soluble epoxide hydrolase in HEK293 cells assessed as DHET productionMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: <1nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: <1nMAssay Description:Inhibition of human soluble epoxide hydrolase in HEK293 cells assessed as DHET productionMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
TargetHydroxycarboxylic acid receptor 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1nM EC50: 6nMpH: 7.4 T: 2°CAssay Description:Membranes were incubated in binding buffer with [5, 6-3H]-niacin in the presence of test compound. After 4 hours at room temperature, reactions were ...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of mouse PrCPMore data for this Ligand-Target Pair
TargetLysosomal Pro-X carboxypeptidase(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of human PrCPMore data for this Ligand-Target Pair

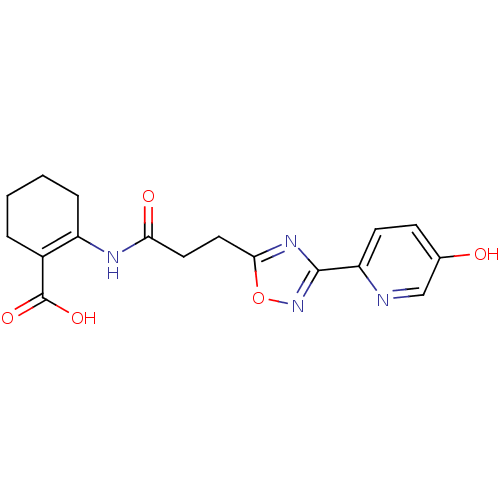
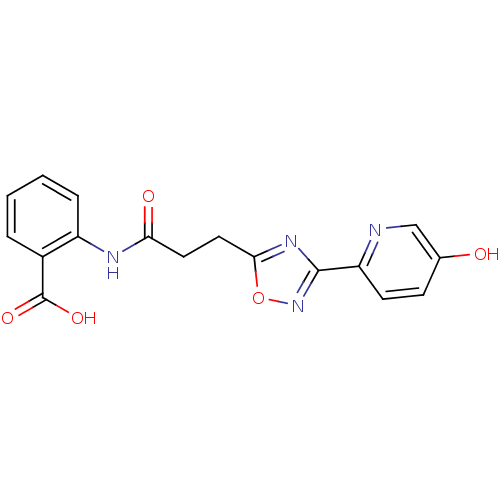
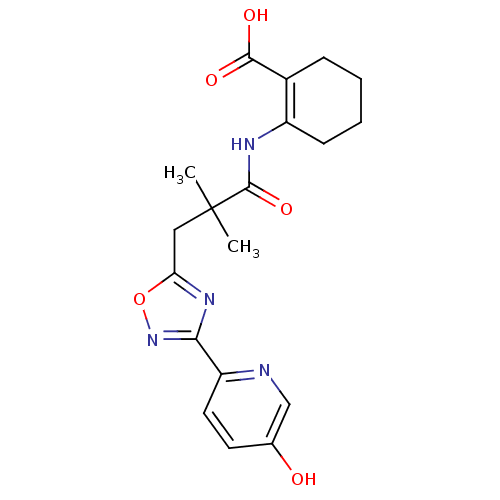
 3D Structure (crystal)
3D Structure (crystal)